Central Oregon Light Colored Rock With Silica

6 Igneous Rocks and Silicate Minerals
KEY CONCEPTS
- Igneous minerals crystallize from a magma to form igneous rocks.
- Magmas have variable compositions giving rise to many different kinds of rocks containing different minerals.
- Cooling rate affects crystal size and rock texture.
- Minerals crystallize in an orderly and predicable way during magma cooling.
- Silicates are the most important minerals in igneous rocks.
- We classify silicates based on the arrangement and ordering of SiO4 tetrahedra within them.
- Different silicate minerals have distinctive properties, atomic arrangements, and origins.
6.1 Magmas and Igneous Rocks
Igneous rocks and minerals form from magma, molten rock that originates beneath Earth's surface. Magma often collects in large magma chambers at depth in Earth, but magma is also mobile and can flow through fissures and sometimes reach the surface. Mid-ocean ridges and subduction zones contain most magmas at or near Earth's surface. Continental rifts and hot spots, places where anomalous heat rises from depth, account for the rest.
Magmas are complex liquids that vary greatly in composition and properties. They have temperatures as great as 1,400 °C and often originate in regions 50 to 200 km deep in Earth. They may be entirely liquid or partially crystalline, containing some crystals of high-temperature minerals such as leucite, olivine, or pyroxene. Because magma has a lower density than the solid upper mantle and crust of Earth, buoyancy moves it upward. The race between upward movement and cooling ultimately determines whether magma becomes an intrusive or extrusive igneous rock.
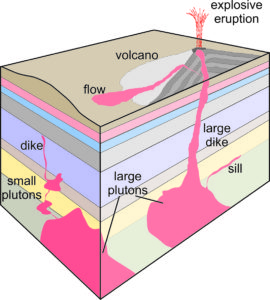
Magma solidifies as an intrusive rock if it crystallizes before it reaches the surface. Intrusive rocks form plutons (a general term given to any intrusive igneous rock body), so geologists sometimes use the terms intrusive and plutonic interchangeably. Figure 6.2 shows some examples of the most common plutonic rock bodies: plutons, dikes, and sills.

This spectacular photo shows a dike revealed by erosion of surrounding sediments. Shiprock, a peak in northwestern New Mexico, is in the background. The tall jagged peak is what remains of the internal plumbing of a volcano that was once present. The dike was created by the same magma that powered the volcano.
Magmas that form intrusive rock bodies are normally surrounded by very warm rocks, so they cool (and crystallize) slowly, perhaps only 1 to several degrees in 1,000 years. In contrast, magmas that reach the surface cool quickly. They may produce lava flows or erupt explosively into the air, ejecting pyroclastic material (ash and other debris of variable size). The result is sometimes just unconsolidated volcanic debris. Typically, though, eruptions produce extrusive rocks (generally called volcanic rocks although a volcano may not be involved).
Cooling rate directly affects grain size of an igneous rock. The common plutonic rock granite contains crystals of quartz and potassium feldspar that are easily seen with the naked eye. In the photo of granite below (Figure 6.4), the quartz is gray and the feldspar is salmon colored. A common volcanic rock, rhyolite (Figure 6.5) has the same minerals, but we need a microscope to see the crystals. The rhyolite in Figure 6.5 is pink because it contains small crystals of pink potassium feldspar. It also contains larger crystals of quartz that appear as dark specks in the rock. Crystals in granites have a long time to form and may grow large. In contrast, volcanic rocks, such as rhyolite, crystallize rapidly because extrusion exposes the lava to water or to the cool atmosphere at the surface of Earth, and crystals are small.
 |  |  |
Sometimes extrusive igneous rocks cool so quickly that no crystals form. This is especially likely to happen if lava meets water. The result is a rock composed of volcanic glass called obsidian. Examples of obsidian appeared in Figures 1.21 (Chapter 1) and Figure 4.5 (Chapter 4). In other cases, different minerals may grow to distinctly different sizes. The result may be a porphyry, a rock in which coarse crystals called phenocrysts are floating in a sea of fine-grained crystals called groundmass. The porphyry shown if Figure 6.6 includes large phenocrysts of both gray quartz (a bit hard to see) and slightly pinkish blocky K-feldspar. The phenocrysts are surrounded by dark fine grained groundmass.
Quickly cooled plutonic rocks may be very fine grained and difficult to tell from volcanic rocks. Some petrologists, therefore, prefer to classify and name igneous rocks based on their grain size rather than their genesis (origin). They divide rocks into those containing very fine grains (aphanitic), rocks containing very coarse grains (phaneritic), and rocks containing combinations of large and small crystals (porphyritic).
6.1.1 Where Do Igneous Rocks Occur?
| Silica (SiO2) Content of Some Common Igneous Rocks | |||
| 45-55 wt% | 55-65 wt% | >65 wt% | |
| volcanic rock | basalt | andesite | rhyolite |
| plutonic rock | gabbro | diorite | granite |
All igneous rocks are made of the same major elements, but elemental ratios vary. The most significant variations are the amounts of silica (SiO2) present (see table). Most igneous rocks contain between 45 and 65 wt% silica. They range from lower-silica basalt and gabbro to higher-silica rhyolite and granite. Other elemental concentrations vary systematically, too. For example, basalt and gabbro contain more iron and magnesium than andesite and diorite, and andesite and diorite contain more iron and magnesium than rhyolite and granite. Other elemental variations are less profound or regular.

Extrusive igneous rocks are found worldwide, and individual varieties are not restricted to a single tectonic setting. Basalt is the most abundant igneous rock in Earth's crust. The oceans cover two-thirds of Earth, and basalt forms at mid-ocean ridges to become the main component of the uppermost oceanic crust. Ocean basalt typically is found in 1/2-km-thick layers that may or may not be covered by sediments. These basalts sometimes form as pillow basalts, like the basalt seen in this photo (Figure 6.7). The pillow structure develops as the basalt is extruded into cold ocean water. Lesser amounts of basalt are found at subduction zones where, in association with andesites, rhyolites, and related rocks, basalts may be part of composite volcanos.

In continental interiors, hot spots and rifts can produce large volumes of basalt flows, in some places creating huge flood basalt provinces such as the Columbia River Flood Basalts of Idaho, Washington, and Oregon. Lava flows may be thick; the basalt cliff in the photo here is about 150 m tall. Because all basalts have similar compositions, they generally contain the same minerals, most importantly plagioclase and pyroxene. Often they contain small or large amounts of volcanic glass as well.

Large volumes of andesite, the most common volcanic rock after basalt, are found with dacite and rhyolite in island arcs and at continental margin subduction zones. The photo in Figure 6.9 shows subduction-related andesite at Fifes Peaks in western Washington. Less commonly, andesite is associated with continental rifts or hot spots. Even more rarely, andesite is found at mid-ocean ridges. Andesite is always rich in plagioclase, and may contain biotite, pyroxene, or hornblende. Quartz and olivine are rare. Rhyolite, always containing quartz and K-feldspar, occurs in the same settings as basalt and andesite but is only common in continental areas. Rhyolites are especially abundant at hot spots such as the Yellowstone Hotspot in northwest Wyoming. They are also associated with continental rifts such as the Rio Grande Rift in southern Colorado and New Mexico, and at subduction zones.
Plutonic igneous rocks, too, occur in many diverse settings. Granites, granodiorites, and related rocks collectively called granitoids, are found worldwide and are major components of the continental crust. They are especially common in Precambrian shields and younger mountain belts, where they form intrusions of all sizes, from small dikes and plutons to large batholiths.


and surrounding peaks near Lone Pine, California. The exposed
rock is part of the Sierra Nevada Batholith.
Batholiths (masses of intrusive rock composed of multiple plutons) form the cores of many mountain ranges. The Sierra Nevada Batholith in California and Nevada, for example, is a mostly granitic mass that crops out, or is just beneath the surface, in a 650 km long region that is 100 to 125 km wide. The photo in Figure 6.10 shows a cross section of the batholith exposed in the vertical face of El Capitan in Yosemite Valley. Look closely at the photo and you can see that there are several different colored rock types. Most are white or salmon colored granite; the darker patches are diorite. Overall, the mineralogy of the Sierra Nevada Batholith is mostly quartz, plagioclase, and potassium feldspar with lesser amounts of biotite and hornblende. Although typically found in shields and mountain belts, batholiths and other granitic rocks are also associated with rifts, spreading centers, and hot spots.

Plutonic rocks span a wide range of compositions. Those that contain less silica than granite, including for example gabbro (equivalent in composition to the most common volcanic rock, basalt), also form plutons of all sizes. They do not, however, form large batholiths. Large volumes of gabbro make up the lower part of oceanic crust. Lesser amounts are found in layered intrusions or associated with continental hot spots and rifts. The Duluth Complex, west and north of Lake Superior, is one of the largest known gabbroic occurrences. The complex is a 16-km thick sill, composed mostly of gabbro with lesser amounts of anorthosite and granitic rocks. The gabbro seen in Figure 6.12 consists of light-colored plagioclase and darker pyroxene.
6.2 Compositions of Igneous Rocks
6.2.1 Mafic and Silicic Magmas
The table below gives chemical analyses for seven different plutonic rocks that formed from seven magmas of different compositions. These compositions include several examples each of very silica-rich and very silica-poor rocks, and cover the range for typical magmas. The most significant variations are the amount of silica (SiO2), and the relative amounts of alkali oxides (Na2O+K2O) compared with CaO. Although compositions cover a wide spectrum, most magmas contain 40 to 75 wt % SiO2. This is because silicon and oxygen are dominant elements in the crust and mantle where magmas originate. Alkali oxide content varies from 2.70 to 14.21 %. Some rare and unusual magma types produce igneous rocks rich in nonsilicate minerals including carbonates or phosphates, but we will not consider them here.
| Compositions of Some Magmas (wt% of oxides) | ||||||||
| oxide | Silicic magmas | Mafic magmas | ||||||
| (Relatively SiO2-rich magmas) | (Relatively SiO2-poor magmas) | |||||||
| higher — Na2O+K2O — lower | higher — Na2O+K2O — lower | |||||||
| alkali granite | granite | tonalite | alkali syenite | syenite | diorite | gabbro | peridotite | |
| SiO2 | 73.86 | 72.08 | 66.15 | 55.48 | 59.41 | 51.86 | 50.78 | 43.54 |
| TiO2 | 0.20 | 0.37 | 0.62 | 0.66 | 0.83 | 1.50 | 1.13 | 0.81 |
| Al2O3 | 13.75 | 13.86 | 15.56 | 21.34 | 17.12 | 16.40 | 15.68 | 3.99 |
| Fe2O3 | 0.78 | 0.86 | 1.36 | 2.42 | 2.19 | 2.73 | 2.26 | 2.51 |
| FeO | 1.13 | 1.67 | 3.42 | 2.00 | 2.83 | 6.97 | 7.41 | 9.84 |
| MnO | 0.05 | 0.06 | 0.08 | 0.19 | 0.08 | 0.18 | 0.18 | 0.21 |
| MgO | 0.26 | 0.52 | 1.94 | 0.57 | 2.02 | 6.12 | 8.35 | 34.02 |
| CaO | 0.72 | 1.33 | 4.65 | 1.98 | 4.06 | 8.40 | 10.85 | 3.46 |
| Na2O | 3.51 | 3.08 | 3.90 | 8.86 | 3.92 | 3.36 | 2.14 | 0.56 |
| K2O | 5.13 | 5.46 | 1.42 | 5.35 | 6.53 | 1.33 | 0.56 | 0.25 |
| H2O | 0.47 | 0.53 | 0.69 | 0.96 | 0.63 | 0.80 | 0.48 | 0.76 |
| P2O5 | 0.14 | 0.18 | 0.21 | 0.19 | 0.38 | 0.35 | 0.18 | 0.05 |
Magmas richest in SiO2, such as alkali granite, granite, and tonalite are generally deficient in MgO. We term such magmas silicic (Si-rich) or felsic (contraction of feldspar and silica). Light-colored minerals dominate felsic rocks, so many geologists use the term felsic to refer to any light-colored igneous rock, even if the chemical composition is unknown. At the other end of the spectrum, magmas with <50 wt % SiO2 are usually rich in MgO and contain more FeO and Fe2O3 than silicic magmas. These include diorite, gabbro, and peridotite. We call them mafic (contraction of the words magnesium and ferric) and, for peridotite, ultramafic. They are usually dark in color. The term intermediate describes rocks with compositions between mafic and silicic (but no intermediate rock analyses are in the table above).

Besides distinctions between mafic, intermediate, and silicic rocks, petrologists often classify igneous rocks based on their alkali (K2O + Na2O) and CaO contents. Alkalic rocks are those with high (K2O + Na2O):CaO ratios. The table above reveals that alkalic rocks can be either silicic or mafic compositions. Overall, though, the most common alkalic rocks are silicic. We grouped alkali syenite and syenite with the other mafic magmas because alkali syenite and syenite are silica-poor compared with silicic magmas, but their most significant distinctions are their high alkali oxide contents compared with CaO content. The photo seen here (Figure 6.13) shows a syenite from Brazil. The main minerals are gray potassium feldspar and lighter colored nepheline. The black mineral is hornblende.
Rocks melt in many places within Earth, and magma compositions reflect the sources. Mid-ocean ridge and ocean hot-spot magmas are mostly mafic; subduction zone magmas are generally silicic to intermediate. Continental rifts produce a variety of magma types. Rocks of different compositions have different melting temperatures because some elements combine to promote melting. Silicon and oxygen, in particular, promote melting because they form very stable molten polymers (long chains of Si and O) that persist even when melted. Silicic minerals, and SiO2-rich rocks, therefore, melt at lower temperatures than mafic minerals and SiO2-poor rocks.
6.2.2 Volatiles

Magmas may also contain gases, liquids, or vapors, collectively called volatiles. H2O and CO2 are the most common volatiles, but volatile compounds of sulfur, chlorine, and several other elements may also be present. Sometimes, these compounds separate from a melt to form bubbles, most commonly in cooling lava, creating empty vesicles as the magma solidifies. Figure 6.14 shows a basalt from Hawaii with vesicles (remnants of gas bubbles) up to several millimeters across. Often, vesicles like these become filled with secondary zeolites or other minerals over time. This rock contains green crystals of olivine, but most of it is dark colored volcanic glass. The specimen also contains a piece of black and white gabbro that was plucked from the lower crust as the magma rose to the surface.

Water is especially important during eruptions because just a small amount of water can produce large amounts of steam and violent eruptions. This is especially true for silicic magmas. The explosive May 18, 1980 eruption of Mt. Saint Helens in Washington is an example. A series of earthquakes and small eruptions began at Mt. Saint Helens in March, 1980. A bulge developed on the mountain's north side and grew for two months as magma moved upward. On May 18, an earthquake caused the north slope to slide away and gas-powered semi-molten magma exploded out of the volcano's side. The eruption produced a column of ash that rose 24 km into the sky. Ash was deposited in a dozen states and two Canadian provinces. Nearly 60 people were killed by what some people call "the most disastrous volcanic eruption in U.S. history." The photo in Figure 6.15 shows one of many smaller steam eruptions that preceded the main event.
6.3 Crystallization of Magmas
6.3.1 Equilibrium Between Crystals and Melt
Every mineral has a characteristic melting temperature. This can be expected to lead to an orderly and predictable sequence of minerals crystallizing as magma cools and solidifies. It does, sort of. Complications arise because some minerals do not crystallize at a single temperature, but instead form from other minerals while reacting with magma. This is called incongruent melting. Further complications arise because minerals together may melt (and crystallize) at lower temperatures than if they if they were alone. This is called eutectic melting. For example, at 1 atm pressure, diopside melts at about 1391 ̊C and anorthite melts at 1553 ̊C. A rock composed of diopside and anorthite, however, will melt at 1278 ̊C.
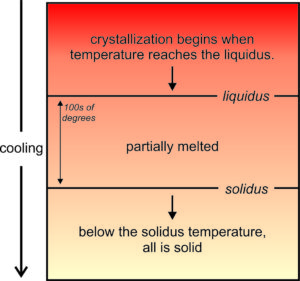
Crystallization of a cooling magma is a step-wise process with some minerals forming before others. So, crystallization occurs over a range of temperature. As seen in Figure 6.16, the first mineral crystals form at a temperature called the liquidus. Crystallization continues until the magma reaches the solidus temperature. Above the liquidus, all is melted. Below the solidus, all is solid. Between the two temperatures a rock is partially melted. The differences between liquidus and solidus temperatures can be 100s of degrees and is different for different composition magmas.
Different magmas produce different minerals, and different minerals crystallize at different temperatures. Consequently, composition is the key factor that determines the temperature at which crystallization begins. Temperatures measured in flowing lavas generally range from 900 to 1,100 °C, with higher temperatures corresponding to basaltic (mafic) lavas and lower temperatures to andesitic or rhyolitic (intermediate to silicic) lavas. For mafic rocks, the first minerals to crystallize are typically olivine, pyroxene, or plagioclase. For silicic rocks, the first crystals may be alkali feldspars or micas. Eventually, a magma completely solidifies, and the last drop of melt crystallizes at the solidus temperature. For some magmas, the solidus is well above 1,000 °C, but for granites and other silicic magmas it may be as low as 700 °C.
6.3.2 Bowen's Reaction Series
N. L. Bowen pioneered in the study of magma crystallization, for which he received the Roebling Medal from the Mineralogical Society of America in 1950. By studying naturally occurring igneous rocks and conducting laboratory experiments, he derived an idealized model for equilibrium crystallization in a magmatic system. We call the model Bowen's reaction series; it is depicted below. Although Bowen's series is a generalization that does not apply in detail to all magma types, it is an excellent model to describe the process of crystallization. Some petrologists have developed more precise models for magmas of specific compositions.

Bowen's reaction series shows the (hypothetical) order in which minerals crystallize from cooling magma. It contains nine mineral names arranged in a Y-shape. We call the left-hand side of the Y the discontinuous side because abrupt changes occur as different minerals crystallize in sequence. We call the right-hand side the continuous side because plagioclase is continually present during crystallization, starting as Ca-rich plagioclase at high temperature and changing to Na-rich plagioclase as cooling progresses. The minerals at the bottom of Bowen's series crystallize at the lowest temperatures. Although not evident from this diagram, just like plagioclase, minerals on the discontinuous side of the series change composition as cooling proceeds. At higher temperatures, for example, olivine usually has a greater magnesium to iron ratio (Mg:Fe) than at lower temperatures.
Mineral crystallization temperatures depend on mineral composition. So, Bowen's series reflects general trends in mineral chemistry. Minerals at the top of the series (olivine, pyroxene, and calcium-rich plagioclase) are mafic (relatively silica poor). Those at the bottom are silicic (relatively silica rich). Silica content is the most significant factor controlling melting and crystallization temperatures. The mafic minerals at the top of the discontinuous series also are deficient in aluminum and alkalis and rich in iron and magnesium, compared with minerals at the bottom.
Bowen's Reaction Series is not meant to imply that all magmas start out crystallizing olivine and end up crystallizing quartz. Consider, for example, a hypothetical magma that is 100% SiO2. It cannot crystallize any minerals in Bowen's reaction series except quartz because it does not contain the necessary elements. It will skip all the other minerals and just form quartz.
On the other hand, if a melt were 100% Mg2SiO4, it would crystallize forsterite (olivine of composition Mg2SiO4) and be completely solidified. These two extreme examples do not exist in nature, but compositions of natural magmas do control the extent to which crystallization follows Bowen's reaction series and which minerals crystallize first (and last). And, although all magmas crystallize different minerals at different temperatures, none follow the complete series.
Bowen's Reaction Series is a convenient way to remember the minerals that are common in different kinds of rocks. Mafic magmas, which crystallize at high temperature, produce rocks containing minerals at the top of the series. Silicic magmas, which crystallize at lower temperatures, produce rocks that contain minerals at the bottom of the series. So mafic rocks such as basalt or gabbro commonly contain olivine, pyroxene and Ca-rich plagioclase. Felsic rocks such as rhyolite or granite are generally rich in K-feldspar and quartz. And, intermediate composition rocks may contain pyroxene, amphibole, or biotite with plagioclase.
6.3.3 Disequilibrium
If magma and minerals remain in equilibrium, different minerals crystallize at different temperatures – sometimes more than one at a time, and mineral compositions change as temperature decreases. Continuous reactions take place as elements move from magma into growing crystals. These relationships are orderly and predictable for a magma of any given composition. But, they are highly dependent on composition. Granite and gabbro, for example, do not crystallize the same minerals, nor do they crystallize at the same temperatures.
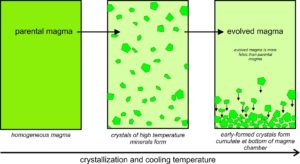
However, mineral crystals and magmas do not always remain in equilibrium during crystallization. Several things may cause disequilibrium. For example, as depicted in Figure 6.18, the first crystals that form may become separated from the rest of the magma if they settle to form a layer called a cumulate at the bottom of the magma chamber. The crystals, then, will not stay in equilibrium with the magma above them. As this happens, an original parental magma changes composition and becomes an evolved magma. Many minerals, including feldspars, pyroxenes, and oxides, can be found in thick, nearly single-mineral cumulates. And, even if a thick cumulate layer does not develop, the composition of the upper part of the magma chamber may not be the same as the lower part. When a melt gets separated from early formed crystals, we call the process partial crystallization, or fractional crystallization.

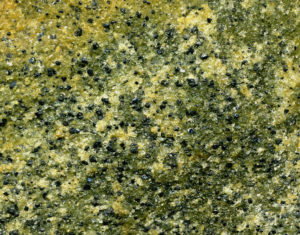
The photos above in figures 6.19 and 6.20 show examples of cumulates. The photo on the left (Figure 6.19) shows dark layers of chromite (chrome-iron oxide) that settled to the bottom of a magma chamber in the Bushveld Complex of South Africa. The light material around the chromite layers is mostly the plagioclase. The Bushveld region is one of the world's foremost mining regions, in part because the natural concentration of chromite makes it easy for the ore to be mined and produced for industrial consumption. Figure 6.20 shows a cumulate from the Stillwater Complex (Montana) that contains both olivine (green) and chromite (black). Like the Bushveld, the Stillwater Complex sees significant mining activity.
●Box 6-1 Layered Mafic Intrusions
Mafic and ultramafic layered intrusions, often called layered mafic complexes, are rare but are found worldwide – generally in old continental interiors. The largest, the Bushveld Complex of South Africa, is more than 66,000 km2. Smaller layered intrusions, such as the Skaergaard Complex (Greenland), may be only 100 km2 or less.
These complexes contain mafic rocks with compositional layering caused by fractional crystallization. They have cumulate layers piled on top of each other. Early formed crystals settled to the bottom of the magma chamber because they were denser than the melt. As the magma cooled and other minerals crystallized, they too settled. Consequently a layered sequence developed with ultramafic (high-temperature) minerals at the bottom and successively more silicic (lower-temperature) minerals on top. New pulses of magma added more layers. Besides silicates, chromite and other oxide minerals accumulate in mafic complexes. Rarely, plagioclase forms a cumulate layer at the top of a magma chamber when it floats on denser magma.

Because of their high metal content and natural separation into concentrated layers, mafic complexes often host rich ore deposits. They are especially important for production of chrome and platinum group metals (ruthenium, rhodium, palladium, osmium, iridium, platinum). The Bushveld complex is, perhaps, the most valuable ore deposit in the world, producing significant amounts of platinum group metals, as well as chromium, iron, tin, titanium, and vanadium.
In the United States, Montana's Stillwater Complex accounts for most of the platinum group metals mined in the western hemisphere. The photo seen in Figure 6.21 shows some of the richest ore at Stillwater. The metallic minerals are pyrrhotite and chalcopyrite that contain significant amounts of platinum, palladium, osmium, and other generally rare elements.

Besides separation of crystals and melt, disequilibrium occurs for other reasons. Sometimes large mineral grains do not remain in equilibrium with a surrounding magma. For example, because diffusion of elements through solid crystals is slow, different parts of large crystals may not have time to maintain equilibrium compositions. In principle, mineral crystals should be homogeneous, but in zoned crystals, such as the tourmaline crystals seen in this photo (Figure 6.22), only part of the crystal remains in equilibrium with the melt as crystallization takes place. Consequently, different zones have different compositions (and sometimes different colors).

Marked chemical zonation often occurs if a magma begins to cool at one depth and then rapidly moves upward to cooler temperatures. The result is often a porphyritic rock with large zoned phenocrysts. The zones may be visible with the naked eye, for example the tourmaline in Figure 6.22, if color or textural variations mirror the compositional variations. Other examples of visibly zoned minerals include the tourmaline in Figure 4.13 (Chapter 4), and the fluorite in Figure 4.37 (Chapter 4). If the zoning is not visible to the naked eye, it may be visible when a crystal is viewed in thin section with a petrographic microscope . The thin section photo seen here (Figure 6.23) shows zoned clinopyroxene. The colors are artifacts and are not real mineral colors. Zoning can also be detected using a scanning electron microscope. See, for example, the electron microscope images of zoned plagioclase in Figure 4.38 (Chapter 4).
6.4 Silicate Minerals
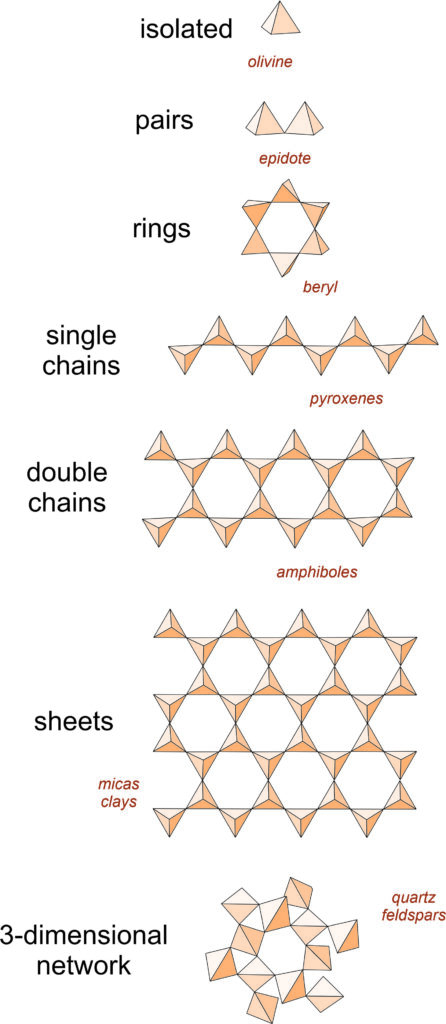
Silicate minerals dominate igneous rocks because silicon and oxygen are the most common elements in magmas. So, in the following discussion we systematically consider the important silicate minerals and groups.
Recall from Chapter 1 that the fundamental building block in silicate minerals is an (SiO4)4- tetrahedron with oxygen at the corners and silicon in the center. Individual tetrahedra bond to other tetrahedra or to cations to make a wide variety of atomic arrangements. The top drawing in this chart (Figure 6.24) shows a single isolated tetrahedron.
Sometimes aluminum substitutes for silicon, so silicate minerals may contain both (SiO4) and (AlO4) tetrahedra. A key property of both silicon and aluminum tetrahedra is that they can share the oxygen anions (O2-) at their corners. When they do this, they form polymers that are various kinds of rings, chains, sheets, or three-dimensional arrangements. Figure 6.24 shows some of the possibilities. (For a slightly different view, see Figure 1.33 in Chapter 1).
In some minerals, (SiO4)4- tetrahedra are not polymerized. They do not share oxygen atoms, and instead are joined together by bonds to other cations. These minerals, called isolated tetrahedra silicates, or island silicates, include most importantly minerals of the olivine group.
In a few minerals, especially minerals of the epidote group, tetrahedra join to form pairs. These are the paired tetrahedral silicates, sometimes called sorosilicates or butterfly silicates. In the pyroxenes and other single chain silicates, tetrahedra link to create zigzag chains. The amphibole minerals are double chain silicates. Micas and clays are sheet silicates. In sheet silicates, tetrahedra share three of their four oxygen with other tetrahedra, creating sheets and minerals with layered atomic arrangements.
Finally, the feldspars and quartz are examples of network silicates, sometimes called framework silicates, in which every oxygen is shared between two tetrahedra, creating a three-dimensional network. The drawing in Figure 6.24 does a poor job of showing this arrangement but the key characteristics are that all oxygen atoms are shared between two tetrahedra and the overall structure is about the same in all directions. The most important framework silicates are quartz and other SiO2 minerals, and the feldspars. In the feldspars, and a related group of minerals called feldspathoids, alkali and alkaline earth elements – mostly Na, K, or Ca – occupy large sites between tetrahedra.
We will start our review of silicate minerals by looking at the SiO2 polymorphs, the feldspars, and the feldspathoids, and then work our way upwards in the figure above, towards minerals with less polymerization.
6.4.1 SiO2 Polymorphs
Silica Minerals
SiO2
α-quartz, β-quartz, stishovite, coesite, tridymite, cristobalite
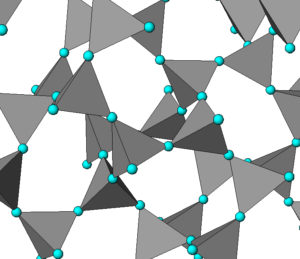
Quartz, like many other minerals, is polymorphic. Mineralogists and chemists have identified more than 10 different silica (SiO2) polymorphs, but some do not occur as minerals. We briefly looked at the most common of these polymorphs in Chapter 4. All the silica minerals (except stishovite) are framework silicates; the differences between the minerals are the angular relationships between the tetrahedra that comprise them. Figure 6.25 shows the atomic arrangement in cristobalite, one of the high-temperature polymorphs. The blue spheres are oxygen atoms and silicon atoms are at the centers of every gray tetrahedron. For some spectacular scanning electron microscope images of several silica polymorphs, see Figure 12.35 in Chapter 12.
 |  |
Common quartz, more properly called low quartz (because it has lower symmetry than high quartz), is the only polymorph stable under normal Earth surface conditions, but it has many different appearances. The anhedral specimen seen in Figure 6.26 has a very common look. But euhedral crystals, such as those shown in Figure 6.27, are also common. Some of the different quartz varieties have specific names, such as milky quartz, rose quartz, Herkimer diamond, amethyst, and citrine. Photos of these were in previous chapters, including:
•Figures 1.8 (Chapter 1) shows a cluster of clear quartz crystals
•Figure 3.4 (Chapter 3) contains a photo of subhedral rose quartz
•Figure 3.32 (Chapter 3) shows a Herkimer diamond
•Figure 3.44 (Chapter 3) show purple amethyst and orange citrine
•Figure 3.61 (Chapter 3) is a photo of anhedral milky quartz
•Figure 4.17 (Chapter 4) shows amethyst in a geode
Silica Polymorph Stability
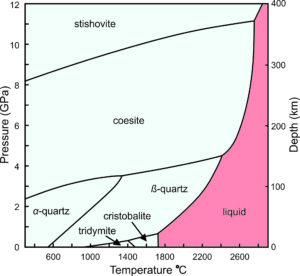
For several decades, petrologists have understood that different silica polymorphs occur in different geological settings because they are stable under different pressure-temperature conditions. This phase diagram (Figure 6.28) shows the stability relationships of some SiO2 polymorphs. The horizontal axis is temperature. The vertical scale on the left gives pressures in gigapascals (GPa), and the scale on the right shows the depths in Earth corresponding to those pressures.
Pressure-temperature (P-T) phase diagrams such as the one seen here show which mineral is stable for any combination of P-T. Solid lines divide pressure-temperature space into stability fields where different polymorphs are stable: stishovite, coesite, α-quartz, β-quartz, tridymite, and cristobalite. For example, at 1400 ̊C and 6 GPa, coesite is the stable polymorph. Low quartz (α-quartz) is the stable phase at low temperature and pressure, including normal Earth surface conditions (which would plot way off to the left on the bottom of this diagram). Low quartz is therefore the most common polymorph. If all rocks maintained and stayed at equilibrium, we would have no samples of any other silica polymorphs to study. However, the other polymorphs sometimes persist and exist metastably at Earth's surface. (Although given enough time, they usually change into low quartz.)
Stishovite and coesite are dense minerals, only stable at very high pressures – pressures not normally encountered on or in Earth's crust. They are usually associated with meteorite impact craters. Tridymite and cristobalite only exist in certain high temperature silicic volcanic rocks. They require temperatures greater than 900 °C to form. Although not shown in this diagram, just like quartz, tridymite and cristobalite have both high- and low-symmetry polymorphs.
The red field in the phase diagram above (Figure 6.28) shows conditions where silica melts. Melting temperature is greatest at high pressure, and is different for the different polymorphs. So, with cooling, molten SiO2 will crystallize to form stishovite at high pressure, coesite at somewhat lower pressure, β-quartz at still lower pressure, and cristobalite at 0.0001 GPa (equivalent to atmospheric pressure at Earth's surface). Consider what happens when a volcano erupts and silica-rich magma cools. If stability is maintained, cristobalite will crystallize at about 1725 °C. With further cooling, it will turn into tridymite at about 1460 °C, then into β-quartz at about 950 °C, and become α-quartz at 573 °C. This happens most of the time but occasionally metastable polymorphs can be found in volcanic rocks.
Quartz in Rocks
Essential minerals are minerals that must be present for a rock to have the name that it does, and quartz is an essential mineral in silicic and intermediate igneous rocks, many sediments, and many metamorphic rocks. Quartz is not normally found in mafic igneous rocks because crystallization of mafic minerals such as olivine or pyroxene generally consumes all silica that is available, so there is none left over to form quartz.

Granites contain essential quartz. In silicic plutonic rocks such as granite, quartz is always associated with K-feldspar, commonly in a mosaic pattern similar to what is seen in this photograph (Figure 6.29). The largest of the grains in this view are pinkish K-feldspar about 1 cm across. Quartz is glassy gray. White plagioclase and black biotite are also present.

Quartz is also an essential mineral in sandstone and some other sedimentary rocks. Quartz is the only mineral present in some sandstones or cherts. But, sandstone may also contain significant amounts of other minerals including feldspar or clay, and sometimes pebbles or rock fragments. Figure 6.30 shows an arkose, a feldspar-containing variety of sandstone, composed of gray quartz, pinkish-orange feldspar, and some obvious quartz pebbles up to 5 mm across.
Quartz cannot exist in rocks containing corundum (Al2O3), because the two minerals would react to form an aluminosilicate mineral of some sort. It cannot exist in rocks containing feldspathoids (leucite, nepheline, or analcime) because quartz and feldspathoids react to give feldspars. For similar reasons, quartz is absent or minor in many alkali-rich igneous rocks and in rocks containing the oxide mineral spinel (MgAl2O4).
 |  |
Quartz may crystallize from silica-saturated water. The photograph on the left above (Figure 6.31) shows white quartz veins cutting through altered granite in Kings Canyon National Park, California. The quartz formed when hot hydrothermal water infiltrated the rock along fractures before the rock was uplifted to the surface and eventually weathered. Temperature need not be high, however, for quartz to precipitate. For example, the amethyst (purple quartz) in the geode shown in Figure 6.32 precipitated from water at low temperature. Figure 4.17 (Chapter 4) shows another example of quartz in a geode.
Quartz Twins
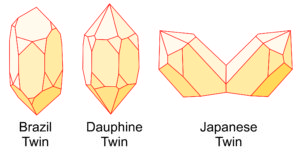
Most quartz crystals are twinned, but the twinning can be impossible to see or easily overlooked. The drawings seen here (Figue 6.33) show the three major ways that quartz twins. Brazil twins and Dauphine twins are penetration twins, and Japanese twins are contact twins. All three are generally growth twins but can also form in other ways. Some quartz crystals exhibit more than one kind of twinning.
Brazil and Dauphine twins are distinguished by symmetry relationships between crystal faces of particular shapes, sometimes by identifying striations (fine lines on crystal faces that developed when the crystal formed), but sometimes are difficult to tell apart. Click on the crystal drawing (right) to see a 15 second video showing Dauphine twinning; the striations on the crystal faces are also apparent. The two large quartz crystals in Figure 6.27, earlier in this chapter, show an example of Japanese twinning.
6.4.2 Feldspars
| Feldspars (Ca,Na,K)(Si,Al)4O8 | |
| Most important end members | |
| anorthite albite orthoclase | CaAl2Si2O8 NaAlSi3O8 KAlSi3O8 |
Feldspars are the most abundant minerals in Earth's crust, in part because they contain six of the seven most abundant elements in the crust. They are widespread and are essential minerals in many igneous, metamorphic, and sedimentary rocks. Feldspars are solid-solution minerals and have the general formula (Ca,Na,K)(Si,Al)4O8. Rarely, they contain significant amounts of other elements such as Ba, Sr, B, or Fe. For most purposes, we consider them to be ternary solutions, which means we can describe their composition in terms of three end members and plot them on diagrams such as the one shown in Figure 6.34 and in Figure 2.22 (Chapter 2). The important feldspar end members are albite (NaAlSi3O8), anorthite (CaAl2Si2O8), and orthoclase (KAlSi3O8).

In Figure 6.34, the blue region shows the compositions of naturally occurring feldspars. Natural feldspars form two distinct series, the alkali feldspar series and plagioclase, both labeled on this triangular diagram. Alkali feldspar, mainly solutions of orthoclase and albite, sometimes contains up to 15 wt % anorthite. Plagioclase, often called plagioclase feldspar, is mostly a solid solution of albite and anorthite, although it may contain up to 10 wt % orthoclase. We call any feldspar with composition near NaAlSi3O8 , albite, and one with composition near CaAl2Si2O8, anorthite, even if other components are present. Intermediate plagioclase compositions are commonly given specific names (labeled in the figure): oligoclase, andesine, labradorite, and bytownite. Labradorite, for example, is plagioclase with composition between 70% anorthite-30% albite and 50% anorthite-50% albite. Labradorite may also contain a small amount of orthoclase. Compositions between plagioclase and alkali feldspar (that would plot in the white part of the triangle) are rare or do not exist.
Confusion sometimes arises because the names of some composition ranges are the same as the names of feldspar end members (albite, anorthite, orthoclase). Orthoclase, for example, is the name given to end member KAlSi3O8. It is also the name we give to any feldspar that is >90 KAlSi3O8. The alkali feldspars, with compositions between NaAlSi3O8 and KAlSi3O8 have not been divided into as many bins as plagioclase feldspars. The term anorthoclase, however, is often used to refer to alkali feldspars with intermediate compositions (10-36% KAlSi3O8 with the rest being mostly NaAlSi3O8).
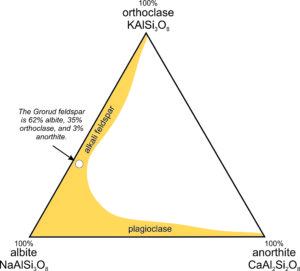
The triangular diagram in Figure 6.35 is similar to the one in Chapter 2 (section 2.5.1); it shows the composition of a feldspar from Grorud, near Oslo, Norway. This feldspar is an example of anorthoclase. It has a composition of about (Ca0.03Na0.62K0.35) (Al1.03Si2.97)O8, so it contains 3 mol % anorthite (CaAl2Si2O8), 62 mol % albite (NaAlSi3O8), and 35 mol % orthoclase (KAlSi3O8). For this feldspar, XAn = 3, XAb = 62, and XOr = 35, where the symbol X stands for mole fraction. Often, we describe the compositions of feldspars by using abbreviations with subscripts. Thus, the Grorud feldspar has composition An3Ab62Or35.
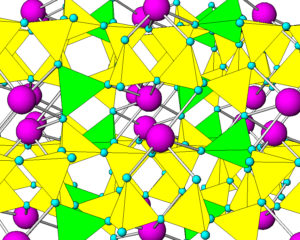
Like quartz, feldspars are framework silicates. Unlike quartz, feldspars contain both SiO4 and AlO4 tetrahedra. As an example, Figure 6.36 shows the atomic arrangement in albite. The purple atoms are sodium, and the yellow and green tetrahedra are (SiO4) and (AlO4), respectively. In all feldspars, Na, K, or Ca occupy spaces between tetrahedra.
Feldspars in Igneous Rocks
Most igneous rocks contain feldspar of some sort, but the kind of feldspar varies with rock composition. In silicic igneous rocks, such as granite, plagioclase is absent or subordinate to K-rich alkali feldspar. If plagioclase is present, it is always albite-rich. Similarly, in mafic rocks, alkali feldspar is not normally present but plagioclase is common. Because mafic rocks contain much more Ca than Na, the plagioclase in them is generally anorthite-rich. Intermediate igneous rocks nearly always contain both feldspars.
 |  |
The two photos above show light colored albite crystals from a classic locality in Austria and anorthite crystals from near Naples, Italy. These are both examples of plagioclase.
Feldspars with compositions close to KAlSi3O8 are generally called K-feldspar, or K-spar. But, K-feldspar has three polymorphs with slightly different atomic arrangements: orthoclase, microcline, and sanidine. Photos of each are shown below in Figures 6.39, 6.40, and 6.41. In these photos the polymorphs have different colors, but color is not a good diagnostic property because the different polymorphs all come in many colors. Note the well developed penetration twins in the sanidine specimen.
 |  |  |
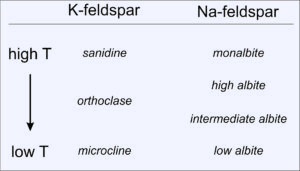
The different K-feldspar polymorphs are stable under different conditions. Sanidine forms in high temperature rocks; orthoclase and microcline form in lower temperature rocks (Figure 6.42). Sanidine commonly crystallizes from silicic lava but may change into orthoclase and, perhaps, microcline if the lava cools relatively slowly. Just about all microcline forms by recrystallization of sanidine or orthoclase.
Like K-feldspar, Na-feldspar forms different polymorphs, with different atomic arrangements, depending on temperature (Figure 6.42). At high temperatures, just below albite's melting point, we call the feldspar monalbite. Three other polymorphs exist at lower temperatures, all generally called albite. Unlike the SiO2 polymorphs, the differences in atomic structure between the feldspar polymorphs are not great and the boundaries on phase diagrams are poorly known. And, when high-temperature feldspars change into low-temperature ones because of cooling or reequilibration at Earth's surface, crystal shape may not change even though atoms move and bonds adjust. Distinguishing between the different Na-feldspar polymorphs can be difficult or impossible without X-ray analysis.
Twinning of Feldspars

Figure 6.43 shows a plagioclase crystal with twin striations across its top. The striations derive from a type of polysynthetic twinning called albite twinning. The atomic arrangements in alternating domains are slightly different and are reflections of each other. See the drawing of albite twinning in Figure 4.43 (Chapter 4) and look at the photo in Figure 4.44 (Chapter 4) for another example of this kind of twinning.
Twinning occurs in all kinds of feldspars but is generally not visible with the naked eye. So, although a feldspar crystal appears homogeneous, it may be composed of two or more twin domains. We most easily see feldspar twinning when using a polarizing microscope to view a feldspar in a thin section. The different domains are visible because they have different optical properties.
 |  |  |
Feldspars twin in several different ways that have different names, for example Carlsbad, Baveno, or Manebach twinning, shown in the three photos above (Figures 6.44, 6.45, and 6.46). The crystals seen in these figures are about 4 cm, 4 cm, and 7 cm tall, respectively. These three kinds of twins are simple twins involving only two twin domains.
Carlsbad twins are penetration twins common in orthoclase, and sometimes in plagioclase. The photo above (left) shows Carlsbad twinning with two orthoclase crystals that appear to have grown through each other. Figures 4.41 (Chapter 4) and 4.42 (Chapter 4) also show Carlsbad twinning.Baveno twins are contact twins that occur most often in orthoclase and microcline. The photo above (center) shows a feldspar with a Baveno twin. A vertical composition plane separates the left and right sides of the crystal. Manebach twins are most common in orthoclase. The photo on the right, above, is a crystal with a Manebach twin. The reentrant angle (two small crystal faces forming a vee shape that points into the crystal) in its top shows where the near-vertical composition plane passes through the crystal. Both Baveno and Manebach twins are rarer than Carlsbad twins. When both are present, Carlsbad and Manebach twinning are diagnostic for orthoclase.
Polysynthetic twins, such as the twins in Figure 6.43 (above), are common in plagioclase and sometimes visible with the naked eye. Albite twinning is one kind; pericline twinning is another. The difference between the two is that domains in albite twins are related by reflection, and domains in pericline twins are related by rotation. Albite twinning is a diagnostic property for plagioclase. Figures 4.43 (Chapter 4)and 4.44 (Chapter 4) show additional examples of albite twinning. Sometimes albite twinning combines with Carlsbad twinning to produce complex crystals, but it is still generally recognizable.

Microcline is the stable K-feldspar polymorph at normal Earth surface conditions. So, other K-feldspars may turn into microcline. Pericline twinning is produced during the polymorphic transformation of sanidine or orthoclase to microcline, and most microcline exhibits both pericline and albite twinning. The combined twinning produces typical microcline twinning, sometimes called cross-hatched twinning or tartan twinning, but we can only see this twinning with a polarizing microscope. Figure 6.47 shows cross-hatched twinning (albite perpendicular to pericline twinning) when viewed with a polarizing microscope. The colors are artifacts and are not the true colors of the domains. The field of view is 2 mm across, and the veins that cut across the field of view contain albite.
Solvus and Miscibility Gaps

Consider alkali feldspars that are solutions of KAlSi3O8 and NaAlSi3O8. At high temperature, as long as they are not so hot that they melt, these feldspars can have any composition between sanidine (KAlSi3O8) and albite (NaAlSi3O8), and may be anorthoclase (part way between the end members). However, as shown in this phase diagram (Figure 6.48), intermediate compositions (anorthoclase) are stable only at high temperatures. At lower temperatures, instead of having one intermediate feldspar, anorthoclase unmixes to produce two separate feldspars, one K-rich and the other Na-rich. This happens because a miscibility gap exists between albite and orthoclase. A miscibility gap is a composition range within which no single mineral is stable under a particular set of pressure-temperature conditions. It is the gray region shown in the phase diagram. The curve above and around the miscibility gap is called the solvus.

Miscibility gaps are common in mineral systems because some elements do not mix well under all conditions. Many mineral series show complete miscibility at high temperatures, meaning that all compositions are stable. At low temperatures, partial or complete immiscibility restricts possible compositions. We might make an analogy to a pot of homemade chicken soup that separates into two compositions (fat and chicken broth) with cooling (Figure 6.49).
We call the process of a single feldspar separating into two compositions exsolution, equivalent to unmixing. If an intermediate-composition alkali feldspar cools rapidly, it may not have time to exsolve. Thus, we have examples of anorthoclase to study. On the other hand, if cooling is slow, the feldspar will unmix. This may result in separate grains of K-feldspar (orthoclase or microcline) and Na-feldspar (albite) developing in a rock. More often, it results in alternating layers or irregular zones of orthoclase and albite within individual crystals. If the layers or zones are planar or nearly so (appearing long and thin in thin section), we call them exsolution lamellae. The lamellae are sometimes visible with the naked eye, but frequently require a microscope to detect. The figure below (Figure 6.50) shows the surface of a once homogeneous feldspar that has separated into two kinds of lamellae, one K-rich and one Na-rich.

If the original feldspar was K-rich, most of the layers will be K-rich feldspar. After it exsolves, it is technically called perthite. If the original feldspar was Na-rich, most of the layers will be Na-rich, and after exsolution, it is technically called antiperthite. For practical purposes, most mineralogists call any exsolved alkali feldspar perthite, because they need compositional information to distinguish perthite from antiperthite. The photo seen in Figure 6.50 shows perthite with salmon colored veinlets of K-feldspar and lighter colored veinlets of Na-feldspar. This is a polished slab of rock; the view is 4.5 cm across.

Because they exsolve, alkali feldspars form what is called a limited solid solution at lower temperatures. Intermediate compositions between orthoclase and albite do not exist. In contrast, intermediate plagioclase between albite and anorthite is common. At high temperatures all compositions are stable and, with cooling, there is generally no significant exsolution. But, several small solvi exist at very low temperature which – if cooling is slow enough – can result in microscopic unmixing. The exsolution may give the feldspars an iridescence or a play of colors that helps to identify them. Labradorite, for example, is identified by its labradorescence, a bluish schiller, caused by exsolution. Figure 6.51 show several examples of labradorite; see also Figure 3.53 (Chapter 3). Because exsolution, if present in plagioclase, is on a very fine scale, mineral properties are relatively homogeneous. So, for most purposes, we can ignore the presence or absence of exsolution in plagioclase.
●Box 6-2 Miscibility Gaps

This figure (6.52) is a slight modification of the temperature-composition (T-X) phase diagram in Figure 6.48.
Consider a feldspar of composition Ab60Or40 (marked with an X on the diagram). At high temperature, it will exist as one alkali feldspar (anorthoclase). As it cools to low temperature it separates into two feldspars beginning at about 730 oC. At 700 oC, for example, the compositions of the two feldspars are shown as red dots. At 600 oC, the two compositions are shown as orange dots. And at 450 oC, the compositions are shown by light blue dots. The compositions start at Ab60Or40 and then follow the solvus toward orthoclase and albite (gray arrows) as temperature decreases. At 450 oC, the composition of the albite-rich feldspar will be 97% NaAlSi3O8, while that of the orthoclase-rich feldspar will be about Ab13Or87. The K-feldspar polymorph stable at low temperature is microcline. So, if the feldspar cools completely, the final result will be a mix of microcline-rich feldspar and nearly pure albite, and because the overall composition is closer to albite than orthoclase, there will be more albite than microcline. The likely result will be anitperthite – a single feldspar crystal that contains exsolution lamellae of different compositions
Exsolution and Ternary Feldspars
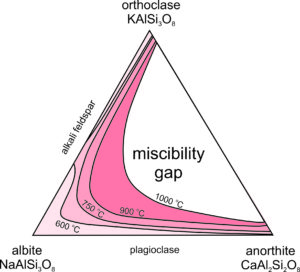
Above, we considered exsolution in alkali feldspars, K-Na feldspars. But, what about ternary feldspars, K-Na-Ca feldspars? As noted, both alkali feldspar and plagioclase exist in a wide range of igneous rocks. But, feldspars with compositions in the middle of the feldspar ternary (shown in white in Figure 6.53) are rarely found because they unmix to form coexisting alkali feldspar and plagioclase. There is a large miscibility gap in the ternary system. At 1000 oC all feldspar compositions, except those that plot in the white region, are stable. But, as temperature decreases the miscibility gap grows wider (show by the temperature contour lines), and if temperature gets as low as 600 oC, feldspar is limited to compositions shown in the lightest pink in this figure.
Feldspar Crystallization – Plagioclase
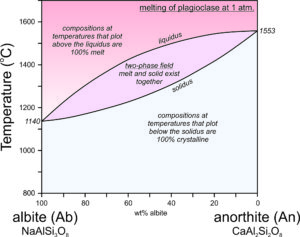
The crystallization temperatures of the two plagioclase end members are different. Albite crystallizes at 1140 oC and anorthite crystallizes at 1553 oC (Figure 6.54). For compositions between albite and anorthite, crystallization is complicated (see Box 6-3) because when an intermediate feldspar begins to crystallize, the first crystal produced is more calcium-rich than the original melt itself. In effect, the anorthite part of the feldspar crystallizes faster than the albite part. If an intermediate plagioclase is partially crystallized, the melt will become more albite-rich. The feldspar that crystallized will therefore have to be more anorthite-rich than the original melt. So, end-member albite and anorthite crystallize congruently. This means they crystallize at specific temperatures and the crystals are the same composition as the melt. But, intermediate composition feldspars crystallize incongruently. They crystallize over a range of temperature and the crystal produced is not the same composition as the melt until crystallization is complete. The temperature at which crystallization begins for a specific composition feldspar is shown by the liquidus curve in the diagram above. Crystallization starts and then continues until temperature reaches the solidus. At temperatures between the liquidus and solidus, in the two-phase field, crystals and melt exist together and are of different compositions.
●Box 6-3 The Plagioclase Phase Diagram and Fractional Crystallization
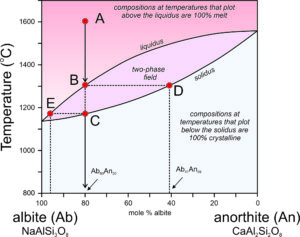
Figure 6.55 is modified from Figure 6.54, the T-X phase diagram describing plagioclase melting and crystallization at 1 atm pressure.
At the highest temperatures, above the liquidus, plagioclase of any composition will be melted. At the lowest temperatures, below the solidus, any plagioclase composition will be entirely crystalline. In between, in the two-phase field, solid crystals and melt will coexist but be of different compositions. This is best described by example.
Point A represents an albite-rich feldspar melt at 1,600 °C. The melt has the same composition as plagioclase that is 80% albite and 20% anorthite (Ab80An20) but is completely molten. As the melt cools, at about 1,300 °C, the first crystals begin to form (point B). A horizontal line at 1,300 °C intersects the liquidus at the melt composition (Point B) and the solidus at the initial crystal composition (Point D). Thus, when crystallization begins, the liquid still has its original composition, but the crystals that start to form are considerably more anorthite-rich than the melt. They have composition Ab41An59.
As the melt continues cooling, crystals continue to form, but they change composition from D to C, following the solidus as temperature decreases. Previously crystallized plagioclase reacts with the melt, so crystal composition changes with cooling and all crystals are homogeneous and have the same composition. A horizontal line can be drawn at any temperature. The intersection of the line with the solidus indicates plagioclase composition and the intersection of the line with the liquidus indicates the composition of the remaining melt. Because the crystals are more anorthite-rich than the melt, the melt becomes more albite-rich as crystallization continues.
As crystallization goes to completion, plagioclase crystals change in composition from Ab41An59 (Point D) to Ab80An20 (the composition of the original melt). Simultaneously, melt composition changes from Ab80An20 to an extremely albite-rich composition (Ab96An04), indicated by Point E on the diagram. At about 1,165 °C, the last drop of melt will crystallize. And, if everything stayed in equilibrium, after complete crystallization, all plagioclase crystals would have the same composition, which must equal that of the original melt.
So, if the crystallization process always went to completion, plagioclase crystals would always end up the same composition as the original melt. However, several things can disrupt this equilibrium. Often crystallization occurs so fast that crystals do not have time to react with the melt as they form. In such cases, the first plagioclase to crystallize is preserved in the centers of large crystals. The crystals are compositionally zoned, the outer zones being more albite-rich. Another complication arises if crystals and melt get separated during the crystallization process. This may occur due to crystal settling, or because remaining melt "squirts off" after the melt is partially solidified. In such cases, crystal-melt equilibrium is not maintained. Both processes are kinds of fractional crystallization that can create melts of different compositions from their parent melts.
Feldspar Crystallization – K-feldspar
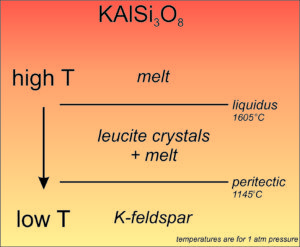
K-rich feldspars can crystallize from a magma, but they exhibit an interesting kind of incongruent crystallization. Consider a melt with composition KAlSi3O8. If it cools, it will eventually reach the liquidus temperature and crystallization will begin. But, as shown here (Figure 6.56), the initial crystals formed will be leucite (KAlSi2O6) instead of K-feldspar (KAlSi3O8). So, as crystallization proceeds, the remaining melt will become more SiO2-rich. Finally, when the temperature reaches what is called the peritectic temperature, the leucite crystals will react with the remaining melt to produce K-feldspar and all will be solid. The difference between liquidus and peritectic temperatures is about 400 °C depending on pressure. If everything stays in equilibrium, we can ignore the temporary presence of leucite crystals. But, sometimes liquid and crystal become separated and leucite may be left behind.
Feldspar Crystallization – Alkali Feldspars

The discussion above is directed at crystallization of K-feldspar, but most alkali feldspars are solid solutions between orthoclase and albite. They are (K,Na)AlSi3O8 feldspars. This complicates crystallization relationships considerably. The phase diagram in Figure 6.57 ignores the incongruent crystallization that would complicate the left side at high temperatures. Assuming that leucite does not crystallize, or that we can ignore it, the alkali feldspar series involves a pair of two-phase fields, similar to the two-phase field on the plagioclase phase diagram (Figure 6.54). There is a temperature minimum (at about 1000 ̊C) for a composition of about Ab60Or40 – this is the lowest temperature where melt can exist. All other compositions are completely crystallized at this temperature.
If melt and crystals stay in equilibrium, when the temperature drops below 1000 ̊C, any composition alkali feldspar will be entirely crystalline and will have the same composition as the original melt. However, the solvus and miscibility gap at lower temperature mean that with more cooling, intermediate composition feldspars will exsolve. They will separate into an albite-rich feldspar and an orthoclase-rich feldspar. The likely result is that perthite will form. See Box 6-2 for further discussion.
6.4.3 Feldspathoids
| Feldspathoids | |
| Some important species | |
| analcime leucite nepheline sodalite | NaAlSi2O6∙H2O KAlSi2O6 NaAlSiO4 Na4(Al3Si3)O12Cl |
The feldspathoid minerals are framework silicates similar to feldspars in many of their properties. They are, however, much less common. Feldspathoids are a family of about a dozen minerals; they are all Na-, K-, and Ca-aluminosilicates. The family is not like other mineral groups, whose members are related by similar atomic arrangements and chemistries. Instead these minerals are grouped together because of their relationship to feldspars. The blue box lists some examples of feldspathoids and their formulas.
 |  |
 |  |
The photos above in Figures 6.58 to 6.61 show the most common feldspathoids. They have compositions that are, for the most part, equivalent to silica-deficient feldspars. These minerals commonly crystallize from magmas that are relatively low in SiO2 or that contain more Na, K, and Al than can fit into feldspars. These minerals have large openings in their atomic arrangements that allow the minerals to contain significant amounts of large anions and molecular anions, including chlorine, carbonate, and sulfate. They often occur with feldspars, and also with mafic minerals including amphiboles, olivine, and pyroxenes, but never with quartz. They are restricted to quartz-free rocks because they will react with quartz to produce feldspars by reactions such as:
blankNaAlSi2O6∙H2O + SiO2 = NaAlSi3O8 + H2O
blankanalcime + quartz = albite + vapor
blankKAlSi2O6 + SiO2 = KAlSi3O8
blankleucite + quartz = orthoclase
blankNaAlSiO4 + 2 SiO2 = NaAlSi3O8
blanknepheline + 2 quartz = albite
Analcime, a zeolite, is often considered to belong to the feldspathoid family. Other important feldspathoids include leucite, nepheline, sodalite, and lazurite. Leucite is a rare mineral found in K-rich volcanic rocks. It is unknown in plutonic, metamorphic, or sedimentary rocks. Nepheline is a common mineral in syenite and other silica-poor volcanic or plutonic igneous rocks. Leucite and nepheline are usually associated with K-feldspar. Sodalite crystallizes from alkali-rich magmas and is also found in some metamorphosed carbonate rocks. Analcime also can crystallize from a magma, but it more commonly forms as a secondary mineral in vugs, cracks, or veins. Occasionally it is found as a secondary mineral in sandstones or tuffs. When it crystallizes from a magma, it is typically associated with olivine, leucite, and perhaps sodalite. When it is secondary, other low-temperature minerals, including zeolites or prehnite, often accompany it.
6.4.4 Micas
| Micas (K,Na,Ca)(Al,Mg,Fe)2-3(Si,Al)4O10(OH)2 general formula: X Y2-3Z4O10(OH)2 | |
| Most important end members | |
| muscovite annite phlogopite | KAl2(AlSi3O10)(OH)2 KFe3(AlSi3O10)(OH)2 KMg3(AlSi3O10)(OH)2 |
| Others | |
| margarite paragonite lepidolite | CaAl2(Al2Si2O10)(OH)2 NaAl2(AlSi3O10)(OH)2 K(Li,Al)2(AlSi3O10)(OH)2 |
We just looked at framework silicates. In those minerals, all four oxygen in SiO4 and AlO4 tetrahedra are shared with another tetrahedron. This is what creates the 3-dimensional framework. In sheet silicates, tetrahedra only share three out of four of their oxygen with adjacent tetrahedra. The most important groups of minerals within the sheet silicate subclass are micas, chlorites, and clays. We will consider only the micas and, to a lesser extent, chlorites, in this chapter and defer discussion of clay minerals to the chapter on sedimentary rocks and minerals.
Micas have the general formula XY2-3Z4O10(OH)2. Some specific formulas are given in the blue box above. The X atoms are most often K and, less commonly Na or Ca, or rarer elements. If the X atom is K or Na, we call the mica a common mica, if it is Ca (e.g., in margarite), we call the mica a brittle mica. Y atoms are generally Al, Mg, or Fe, and less often Mn, Cr, Ti, or Li. Z atoms are mostly Si, with lesser amounts of Al, and even lesser amounts of Fe or Ti. Additionally, some Cl or F may substitute for (OH). So, micas have highly variable chemistry. Structurally, micas are classified as dioctahedral if the number of Y atoms is 2, and trioctahedral if the number of Y atoms is 3. Intermediate micas, part way between dioctahedral and trioctahedral, do not exist. Of the micas listed in the box, muscovite, margarite, paragonite, and lepidolite are all dioctahedral; annite and phlogopite are trioctahedral. The photos below show examples common micas.
 |  |  |
 |  |  |
By far, the most abundant micas are muscovite and biotite. Biotite is the name we use for micas that are mosty solid solutions of phlogopite and annite. Muscovite is more common than biotite, but both occur in a wide variety of igneous and metamorphic rocks. Muscovite (Figure 6.62, above) is dioctahedral and is usually close to the end-member composition KAl2(AlSi3)O10(OH)2. Without chemical analysis, it is sometimes hard to distinguish it from other silvery dioctahedral micas such as margarite (Figure 6.65), paragonite (Figure 6.66), and lepidolite (Figure 6.67). Biotite (Figure 6.63), K(Fe,Mg)3(AlSi3)O10(OH)2, is trioctahedral and may have any composition between the two end members annite and phlogopite (see formulas in the blue box above). Biotite sometimes incorporates a very small amount of muscovite as well.
Micas react to form clays and other minerals when exposed to prolonged weathering, and are therefore absent from most sedimentary rocks. Biotite, and especially muscovite, are relatively Al- and Si-rich compared with many other igneous minerals. Muscovite, therefore, is found in silicic igneous rocks such as granite, but rarely in rocks of intermediate or mafic composition. Biotite is found in rocks ranging from granitic to mafic composition. Phlogopite is occasionally found in ultramafic rocks.
The name annite refers only to the ideal iron-rich mica end member, but the name phlogopite is often used in a more general sense to describe any magnesium-rich biotite. Phlogopite is often brown, compared to the more common black color of biotite, so many geologists use the names muscovite, phlogopite, and biotite in a generic sense to refer to silvery, brownish, and black micas, respectively.
Besides Al-Fe-Mg substitutions, other substitutions, such as F– substituting for OH–, occur in micas, but they are generally minor. Lepidolite, an especially lithium-rich mica similar to muscovite, is an exception. It is a common large and euhedral mineral in pegmatites, and is often associated with the lithium-aluminum pyroxene, spodumene. The photo of lepidolite in Figure 6.67 has the typical lilac color of this mineral, but lepidolite may be yellow or other colors as well.
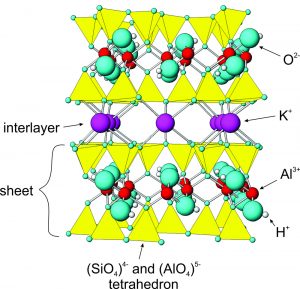
The drawing in Figure 6.68 shows the atomic arrangement in muscovite. The arrangement is just about the same in all micas, and other sheet silicates have atomic structures that are closely related to muscovite's. Micas contain sheets (layers) of (Si,Al)O4 tetrahedra with alkalis and other metals between. Most micas, like muscovite, contain K+ as an interlayer cation between the tetrahedral layers. Depending on the mica species, Fe2+ , Mg2+ , and Al3+ occupy positions between the apices of the tetrahedra. Within the tetrahedra themselves, Al3+ substitutes for some Si4+.
Bonding within a sheet (O2- bonded to Si4+ and Al3+ in muscovite) is much stronger than bonding between sheets (K+ bonded to O2- in muscovite). Consequently, well-developed basal cleavage (planar and sheet-like) characterizes mica and most other sheet silicates. See the photos above in Figures 6.62 to 6.67 and also the biotite in Figure 3.59 (Chapter 3)

The name chlorite is often used in a general way to refer to any greenish mica. But, strictly speaking, chlorite is a group of minerals consisting of a number of different species that are difficult to distinguish from each other. Chlorite compositions can be described by the simplified formula (Mg,Fe)3(Si,Al)4O10(OH)2∙n(Mg,Fe)3(OH)6. The photo in Figure 6.69 shows a rare bluish example of chlorite.
Chlorite's atomic arrangement is similar to the arrangement in micas but the interlayer site contains (Mg,Fe)(OH)6 instead of K. Clinochlore – (Mg5Al)(AlSi)3O10(OH)8 – is perhaps the most important chlorite end member. Chlorite group minerals are stable over a wide range of conditions, generally occurring in low to medium-grade metamorphic rocks. They are also common as alteration products in igneous rocks, where they form from biotite, pyroxene, amphibole, and other mafic minerals.
6.4.5 Chain Silicates
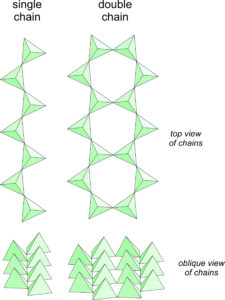
In micas, and other sheet silicates, silicon tetrahedra are polymerized to form sheets. In chain silicates, the tetrahedra polymerize to form chains. This is done in two ways, shown in Figure 6.70. Single chain silicates have tetrahedra that zig-zag back and forth. Double chain silicates also involve tetrahedra that zig-zag back and forth, but the chains come in pairs, and an oxygen anion, called a bridging oxygen, links chains together. A few relatively obscure minerals have chains that are wider and more complex than double chains – the are part way to being sheet silicates. Pyroxenes and amphiboles are the two most important groups of minerals in the chain silicate subclass. In fact, they are almost the only minerals in the subclass.
All chain silicates contain single or double chains of tetrahedra linked by cations. Pyroxenes, and their cousins pyroxenoids, are both single chain silicates and have closely related atomic arrangements. Amphiboles, such as tremolite or hornblende, are double chain silicates. Because two oxygen are shared with other tetrahedra, pyroxenes have formulas that contain (SiO3) or (Si2O6). Less obvious, but equally valid, is that amphibole formulas contain (Si8O22). In both pyroxenes and amphiboles, however, Al may substitute for significant amounts of Si.
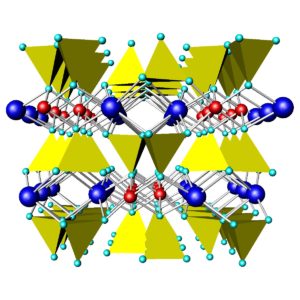 | 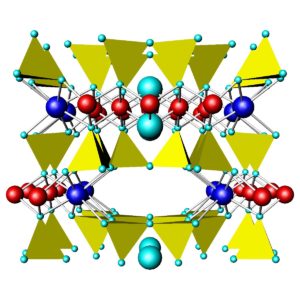 |
Figures 6.71 and 6.72 above compare the atomic arrangements in diopside and tremolite. Diopside is a Ca-Mg pyroxene (single chain), and tremolite is a Ca-Mg amphibole (double chain). In both figures, the view is down the chain direction; the yellow tetrahedra are the (SiO4) units that form the chains. In pyroxenes, the linking cations (blue and red in these figures) occupy two distinct kinds of sites between the chains; in amphiboles, they occupy three kinds of sites between the chains. In both pyroxenes and amphiboles, two interchain sites (blue) are significantly larger than the others. In diopside and tremolite, the large sites contain Ca and the smaller sites contain Mg. The large light blue spheres in the tremolite structure are hydroxyl (OH) groups. Compare these figures with the one above (Figure 6.70) to get a different perspective.
Pyroxenes are anhydrous minerals having simple formulas compared with amphiboles, all of which are hydrous. Still, the similarity between the structures of pyroxenes and amphiboles results in some similarity in physical properties. For example, unless cleavage is visible, it can be difficult to distinguish dark-colored pyroxenes from dark-colored amphiboles.
6.4.6 Pyroxenes
| Pyroxenes (Ca,Na,Mg,Fe)(Mg,Fe,Al)(Si,Al)2O6 | |
| Most important end members | |
| wollastonite ferrosilite enstatite diopside hedenbergite | Ca2Si2O6 Fe2Si2O6 Mg2Si2O6 CaMgSi2O6 CaFeSi2O6 |
Pyroxenes contain many different elements, but all pyroxenes have the general formula shown in the blue box. The most common pyroxenes are close to Ca(Mg,Fe)Si2O6 or (Mg,Fe)2Si2O6 in composition. So, we can describe the chemistries of most pyroxenes in terms of the five end members listed in the box. The formulas for wollastonite, ferrosilite and enstatite have been written with six oxygen, but they could be written with three. We generally use six to keep formulas consistent with other pyroxenes such as diopside and hedenbergite and also because Ca, Fe, and Mg occupy two distinctly different atomic sites in pyroxenes. Distinguishing the different pyroxene species can be challenging. In the absence of a chemical analysis, black colored pyroxenes are often called augite.


Some pyroxenes contain minor to appreciable amounts of Na and Al. Na may substitute for Ca, Mg, or Fe. Al can substitute for Ca, Mg, or Fe, and also for Si. An end-member pyroxene, jadeite, has the formula NaAlSi2O6. It is found in high-pressure metamorphic rocks and is one of two types of jade that are sometimes prized as gemstones. (The other is an amphibole). Other Na-bearing pyroxenes such as acmite (NaFeSi2O6) are found in some igneous rocks. The photo in Figure 6.73 shows a sample of massive green jadeite from central California. It is about 5 cm across.
Li, Cr, and Ti also are sometimes in pyroxene, but are normally minor elements. However, spodumene (LiAlSi2O6), is a major mineral in some pegmatites. The photo seen here (Figure 6.74) shows a yellow spodumene crystal about 2 cm tall. Like jadeite, spodumene, especially the lilac-colored variety called kunzite, can be a valuable gemstone.
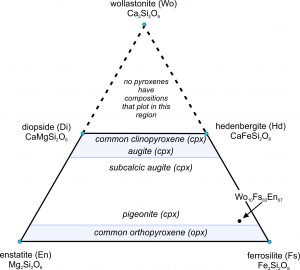
The ternary diagram in Figure 6.75 is a wollastonite-ferrosilite-enstatite triangle with the important pyroxene end members labeled (enstatite, ferrosilite, diopside, hedenbergite, and wollastonite). The mineral wollastonite, although used as an end member to describe pyroxene compositions, is not a pyroxene. It has a slightly different atomic arrangement and belongs to the pyroxenoid group. (In pyroxenoids, the tetrahedral chains do not zig-zag back and forth as regularly as is shown in Figure 6.70.)
As with the feldspars, we use abbreviations to describe pyroxene compositions. For example, a pyroxene of composition Wo10Fs83En07 is 10% CaSiO3, 83% FeSiO3, and 7% MgSiO3. It has the formula (Ca0.10Fe0.83Mg0.07)SiO3, equivalent to (Ca0.20Fe1.66Mg0.14)Si2O6. This composition is plotted as a dot near the ferrosilite corner of the triangle in Figure 6.75.
We call the lines between end members on ternary diagrams joins. The four-sided polyhedron bounded on the top by the diopside-hedenbergite join, and on the bottom by the enstatite-ferrosilite join, is the pyroxene quadrilateral. It encompasses the compositions of all natural Ca-Mg-Fe pyroxenes.
Natural pyroxenes fall into two main series, distinguished by slightly different atomic arrangements and different crystal shapes: the orthopyroxene series (Opx) and the clinopyroxene series (Cpx). Orthopyroxenes, predominantly solid solutions of the end members ferrosilite and enstatite, have the general formula (Mg,Fe)2Si2O6. The photo on the left below (Figure 6.76) shows green enstatite crystals (orthopyroxene) in a volcanic rock from near Crater Lake National Park, Oregon. Natural orthopyroxenes often contain small amounts of CaSiO3. So, their compositions do not plot on the bottom of the quadrilateral, but slightly above.
 |  |
The photo on the right above (Figure 6.77) shows an example of clinopyroxene, green diopside crystals in a metamorphosed limestone. Most clinopyroxene, is predominantly a solution of diopside and hedenbergite, and so has the general formula Ca(Mg,Fe)Si2O6. But natural pyroxenes may be somewhat deficient in CaSiO3. This give them a composition that does not plot on the diopside-hedenbergite join but, instead, plots within the pyroxene quadrilateral. A less common kind of clinopyroxene, pigeonite, has composition close to orthopyroxene. No pyroxenes have compositions more calcic than diopside or hedenbergite because Ca is limited to only the larger two of the four sites between the tetrahedral chains in pyroxenes.

We call pyroxenes with compositions that plot within the quadrilateral augite, subcalcic augite, or pigeonite (see Figure 6.75) depending on their composition. Augite and subcalcic augite are clinopyroxenes with Ca : (Mg + Fe) ratios less than 1. Pigeonites, first found at Pigeon Point, Minnesota, are very high-temperature pyroxenes that only form in quickly cooled volcanic rocks. Figure 6.78 shows an example of large black augite crystals.
Because orthopyroxenes and clinopyroxenes have slightly different atomic arrangements there is a complex solvus between them. At high temperatures, intermediate composition pyroxenes – those that plot within the quadrilateral can be stable. At low temperatures most of the pyroxene quadrilateral is taken up by a large miscibility gap, so intermediate composition pyroxenes are unstable.
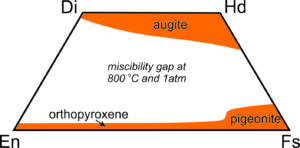
Figure 6.79 shows the gap (in white) at 800 ̊C. Because of this gap, homogeneous pyroxenes with intermediate compositions are only found in some rare high-temperature rocks. At lower temperatures they tend to "unmix." Analogous to the feldspars, a homogeneous high-temperature pyroxene of intermediate composition may become unstable and separate into two pyroxenes at low temperature. This sometimes leads to exsolution and textures similar to perthitic feldspars.
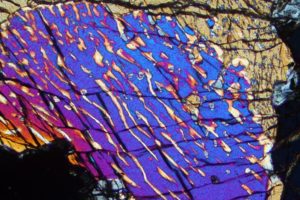
The photo in Figure 6.80, taken through the lens of a petrographic microscope, shows exsolution in pyroxene. The colors are artifacts and not the true color of the minerals. An original pyroxene (subcalcic augite) unmixed upon cooling, producing exsolution lamellae (the stripes) and the texture we see here. The blue-red lamellae are clinopyroxene and the light colored lamellae are orthopyroxene. The compositions of both lamellae depend on the temperature at which exsolution occurred (Box 6-4). So, the compositions of exsolved pyroxene (and feldspar) lamellae are sometimes used as geothermometers to learn the temperature at which a rock equilibrated.
●Box 6-4 Diopside-Enstatite Solvus and Geothermometry
The miscibility gap between orthopyroxene (Opx) and clinopyroxene (Cpx) is sometimes used to calculate the temperature at which igneous or metamorphic rocks formed. Petrologists call such mineral systems geothermometers. Geothermometers are based on a fundamental consequence of thermodynamics: at high temperatures, solid-solution minerals may have intermediate compositions, but at low temperatures many solid solutions unmix so that compositions are relatively close to end members. Figure 6.80 (above) shows an example of a single pyroxene grain that has unmixed to become two pyroxenes.
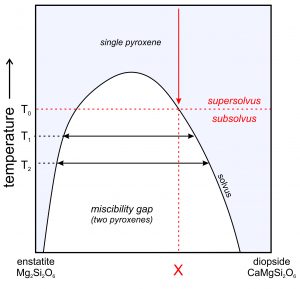
Figure 6.81 is a schematic showing the Opx-Cpx gap between enstatite and diopside. The gap narrows at high temperature and does not extend to the very high temperatures where magmas crystallize and pyroxenes first form. Consequently, pyroxenes of any composition can crystallize from a magma. But, with cooling, many pyroxenes will unmix, producing compositions closer to end member enstatite and diopside than the original pyroxene.
A pyroxene of composition X will be stable at temperatures above T0, but if it crystallizes or equilibrates at lower temperature, it will unmix to form two pyroxenes. So, as temperature decreases from supersolvus to subsolvus (at T0), unmixing will produce separate grains of Opx and Cpx in the same rock, or it may produce single grains of pyroxene containing blebs (small inclusions with exsolved compositions in a larger host) or exsolution lamellae of different compositions. As temperature decreases from T0 to T1 to T2, the coexisting pyroxenes will become closer to end member enstatite and diopside. By analyzing the compositions of coexisting Opx and Cpx, petrologists can estimate the temperature of equilibration.
Pyroxenes are not the only minerals that can be used as geothermometers. Feldspars, carbonates, and others can serve the same purpose. Geothermometry is not, however, always simple or straightforward. Many things besides temperature affect the compositions of coexisting minerals. Among others, petrologists must be concerned with the effects of pressure, of minor elements in minerals, and of disequilibrium.
6.4.7 Amphiboles
| Amphiboles (K,Na)0-1(Ca,Na,Mg)2(Mg,Fe,Al)5(Si,Al)8O22(OH)2 | |
| Most important end members | |
| tremolite ferroactinolite anthophyllite cummingtonite grunerite glaucophane | Ca2Mg5Si8O22(OH)2 Ca2Fe5Si8O22(OH)2 Mg7Si8O22(OH)2 Mg7Si8O22(OH)2 Fe7Si8O22(OH)2 Na2Mg3Al2Si8O22(OH)2 |
Amphiboles and pyroxenes are closely related minerals that commonly coexist. Both are chain silicates, but the atomic arrangement in amphiboles is more complex than in pyroxenes. Like pyroxenes, amphibole chemistry is highly variable and yields many different end member formulas. Just a few are listed in the blue box. Also, like the pyroxenes, amphiboles fall into two main series: the orthoamphibole series and theclinoamphibole series. Note that, in the box we have listed Mg7Si8O22(OH)2 two times. Anthophyllite is an orthoamphibole and cummingtonite is a clinoamphibole; the two are polymorphs. The lengthy generic formula at the top of the blue box describes hornblende, the most common kind of amphibole. The formula is complicated, but really does not reflect all possible compositional variations. Besides the elements listed, Mn and Ti are major components in some amphiboles, and F or Cl can replace (OH) in others. Note that hornblende contains variable amounts of K or Na (indicated by the subscript "0-1″) that are missing from all the other amphiboles listed in the box.
Figure 6.82 shows an amphibole quadrilateral, similar to the pyroxene quadrilateral we saw before. The quadrilateral contains a large miscibility gap between the calcic amphiboles and the calcium-poor amphiboles, analogous to the one between clinopyroxene and orthopyroxene. The quadrilateral includes the compositions of all Ca-Mg-Fe amphiboles. Along the base of the diagram, the figure is complicated because Ca-free amphiboles form two distinct series, having different atomic arrangements: the anthophyllite series and the cummingtonite-grunerite series.

We call the most Mg-rich amphiboles anthophyllite or, if they contain appreciable amounts of iron, ferroanthophyllite. Figure 6.83 shows a group of anthophyllite needles in a 4 cm wide specimen. The cummingtonite- grunerite series contains other intermediate and Fe-rich amphiboles that are poor in calcium. We give aluminous anthophyllite (not shown in the amphibole quadrilateral) the name gedrite.
 |  |  |
Calcic amphiboles can have any composition between an Mg end member (tremolite) and an Fe end member (ferroactinolite). We call compositions between actinolite. The left and center photos above, Figures 6.84 and Figure 6.85, show typical actinolite and tremolite. Actinolite is generally green, and tremolite is white or light colored. The bladed crystals form because of the chain nature of atomic arrangements.

The amphibole quadrilateral depicts variations in Ca, Mg, and Fe content well, but many amphiboles, especially hornblendes, contain K, Na, Al, Ti, and other elements in significant amounts. Additionally, hornblende, the most common amphibole, is a complex mineral that contains many possible elements. It does not have the same number of atoms in its formula as quadrilateral amphiboles. Although many amphibole varieties have specific names, without chemical analyses, telling different amphiboles apart is difficult. So, the name hornblende is commonly used to refer to any black amphibole. The photo in Figure 6.86 shows three pieces of massive hornblende. This photo (Figure 6.87) shows an outcrop of metamorphic rock called amphibolite. All the dark mineral grains are hornblende.
Many amphibole end members have analogs in the pyroxene group; we compare some of the more important end members in the tables below.
|
| ||||||||
Amphiboles are important and essential minerals in many kinds of igneous rocks. For example, hornblende is widespread. It is common in granitic rocks but is even more common in rocks of intermediate to mafic composition. Amphiboles also exist in many metamorphic rocks, including marbles and metamorphosed mafic igneous rocks. They are especially common in high-temperature amphibolites like the one seen above in Figure 6.87. Amphibolites always contain amphibole and plagioclase, and generally other minerals including biotite, epidote, or garnet.

Figure 6.88 shows glaucophane, a blue sodic amphibole, that has the general formula Na2Al2Si8O22(OH)2. Its presence is often associated with rocks formed in subduction zones under high pressures and moderate temperatures. Other Ca-free amphiboles (anthophyllite, gedrite, cummingtonite, and grunerite) are found in metamorphic rocks and occasionally in extrusive igneous rocks. Tremolite is common in high-temperature marbles. As with some other silicates found in marbles, identifying it may be difficult because of its inconspicuous white color. See Figure 6.85, above.
6.4.8 Ring Silicates and Paired Tetrahedral Silicates
| Ring Silicates and Paired Tetrahedral Silicates | |
| The most common ring silicates | |
| beryl tourmaline cordierite | Be3Al2Si6O18 (Na,Ca)(Fe,Mg,Al,Li)3Al6(BO3)3Si6O18(OH)4 (Mg,Fe)2Al3(Si5AlO18) |
| The most common paired tetrahedral silicates | |
| epidote zoisite lawsonite | Ca2(Al2,Fe)(SiO4)(Si2O7)O(OH) Ca2Al3(SiO4)(Si2O7)O(OH) CaAl2Si2O7 |
Ring silicates, generally containing rings of six silicon tetrahedra (see Figure 6.24), are relatively rare. Beryl, tourmaline and cordierite are the only common examples. A few obscure and uncommon minerals have rings of three tetrahedra.
Beryl, tourmaline, and cordierite are sometimes beautiful gemstones. But, the more common varieties are often rather drab. Figures 6.89 and 6.90 show examples of common beryl and tourmaline. Because these mineral have atoms arranged in hexagonal rings, their crystals often exhibit hexagonal symmetry, as can be seen in Figure 6.89. Figure 6.108, later in this chapter, shows an example of gemmy tourmaline. It is similar to one we saw in Figure 4.13 (Chapter 4). Figures 4.1 and 4.30 (Chapter 4) show examples of aquamarine and emerald, both gemmy varieties of beryl.
 |  |
Paired tetrahedral silicates contain linked pairs of silicon tetrahedra that create an Si2O7 group (see Figure 6.24 earlier in this chapter). Epidote, zoisite, and lawsonite are the most common examples. But, epidote and zoisite contain paired tetrahedra and tetrahedra in other configurations as well. The photos, in Figures 6.91, 6.92, and 6.93 show beautiful examples of these minerals. More common specimens are not so pretty.
 |  |  |
Beryl and tourmaline contain beryllium and boron, respectively – these elements are generally quite rare. But, they concentrate in some pegmatites, and that is where most beryl and tourmaline are found. Cordierite and zoisite are both common metamorphic minerals. Lawsonite is a rare high pressure metamorphic mineral associated with metamorphism in subduction zones. The chemistry of tourmaline is highly variable, but the other minerals are generally close to the end member compositions listed in the blue box above.
6.4.9 Olivine Group Minerals
| Olivine Group Minerals (Mg,Fe,Ca,Mn)SiO4 | |
| Olivine end members | |
| forsterite fayalite tephroite calcio-olivine | Mg2SiO4 Fe2SiO4 Mn2SiO4 Ca2SiO4 |
| Other olivine group minerals | |
| larnite monticellite | Ca2SiO4 CaMgSiO4 |
| Related minerals | |
| zircon topaz | ZrSiO4 Al2SiO4(F,OH)2 |
Olivine group minerals, belonging to the isolated tetrahedra silicate subclass, all have similar atomic arrangements. By far, the most important mineral of this group is called olivine. In contrast with some of the other silicates previously discussed, olivine chemistry is quite simple. Its general formula is (Mg,Fe,Ca,Mn)2SiO4 but often we omit Mn and Ca because they are normally minor components. The most important end members are listed in the blue box.
Besides olivine, o ther minerals of the group include larnite, a polymorph of calcio-olivine, and monticellite. Both are rare. Zircon and topaz, have different atomic arrangements than the olivine minerals and so are not members of the group. They are, however, other important isolated tetrahedra silicates and so are listed here.
Natural olivines are generally solid solutions of fayalite and forsterite with only minor tephroite and calcio-olivine. We usually use abbreviations and subscripts to indicate olivine compositions. Fo88Fa09Te02La01, for example, refers to an olivine of composition (Mg0.88Fe0.09Mn0.02Ca0.01)2SiO4.
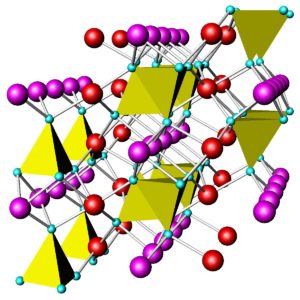
In olivine group minerals, SiO4 tetrahedra do not share oxygen but, instead, are linked via bonds to other cations. Figure 6.94 shows an example. The red and purple spheres are mostly Fe and Mg in common olivine. In monticellite, the red spheres are Ca and the purple spheres are Mg. Because the silicon tetrahedra are isolated, bond strength is about the same in all directions. Consequently, olivine has no direction of good cleavage.
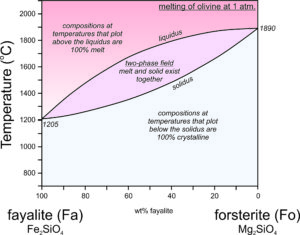
Olivine is primarily an igneous mineral, crystallizing from high-temperature magmas. Its two most important end members, forsterite and fayalite, melt at different temperatures, and olivine's crystallization behavior is similar to that of plagioclase. Figure 6.95 is a phase diagram that describes crystallization of forsterite-fayalite olivines, and the two-phase field between the liquidus and solidus. Compare this with Figure 6.54 (plagioclase phase diagram); they are nearly identical. Partial crystallization of olivine-rich melts leads to crystals that are more Mg-rich than the parent melt, and partial melting of olivine crystals leads to melts that are more Fe-rich than the parent crystals. If the melt and crystals become separated, the Fe-rich melt will move upwards and carry iron from depth toward Earth's surface. Magnesium will be left behind. This is one reason why Earth's crust is more Fe-rich than the mantle.
 |  |
Olivine occurs predominantly in mafic and ultramafic igneous rocks. Its very mafic composition restricts its occurrence, and other mafic minerals containing the same elements with additional silicon are more abundant. In some basalts Mg-rich olivine forms large green phenocrysts in a fine-grained plagioclase-pyroxene groundmass; Figure 6.96 shows an example from the Canary Islands.
Mg-rich olivine is never found in felsic rocks, but Fe-rich olivine is occasionally found in some granites. Olivine occurs in some metamorphic rocks, including marble and metamorphosed igneous rocks. Olivine also is found in mantle xenoliths – samples of the mantle that have been carried to the surface by magmas. The photo in Figure 6.97 is dark basalt that contains a mostly green xenolith. The green colored crystals in the xenolith are olivine. Dark brown orthopyroxene is also present, but in lesser amounts.
6.4.10 Summary: Silicate Minerals and Igneous Rocks
The table below lists the silicate minerals just considered. Quartz, at the very top of the table, has an Si:O ratio of 1:2. The ratio decreases downward in the table, and olivine, at the bottom of the list, has an Si:O ratio of 1:4. This reflects the fact that quartz is a felsic mineral and olivine is a mafic mineral. Things are a bit more complicated for minerals other than quartz and olivine because some Al commonly substitutes for some Si. But the ratio of Si plus the substituting Al (collectively called T) to oxygen increases from 1:2 at the top of the chart to 1:4 at the bottom. The key parts of the mineral formulae that show this are highlighted in blue in the table.
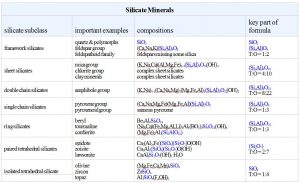
The left hand column in this table lists the silicate subclass, and the right hand column shows the ratio Si:O, or (Si,Al):O in a formula. So, the ratio tells us whether a silicate mineral is a framework, sheet, double chain, etc. All double chain silicates, for example, contain (Si,Al)8O22 in their formulas. Paired tetrahedral silicates contain Si2O7 in their formulas. Note that a few minerals, including epidote and zoisite, belong to more than one silicate subclass. Their formulas contain both SiO4 and Si2O7 and so they are partly paired tetrahedral silicates and partly isolated tetrahedral silicates.
Two caveats: Sometimes formulas are simplified by dividing by two or some other factor. For example, the pyroxene enstatite has formula Mg2Si2O6 but can also be written MgSiO3. It is the ratio (2:6 or 1:3) of Si:O tells us it is a single chain silicate. Also, sometimes subscripts are combined in formulas such as zoisite's – it can be written Ca2Al3Si3O12(OH), which gives no hint about the way tetrahedra are polymerized.
| Accessory Minerals in Igneous Rocks | ||
| magnetite ilmenite apatite zircon titanite pyrite pyrrhotite allanite tourmaline sodalite fluorite | Fe3O4 FeTiO3 Ca5(PO4)3(OH,F,Cl) ZrSiO4 CaTiSiO5 FeS2 Fe1-xS (Ca,Ce)2(Al,Fe)3Si3O12(OH) see formula in table above Na3Al3Si3O12•NaCl CaF2 | |
This chapter focused on silicates, but igneous rocks often contain other minerals. The most common are listed in the table seen here. Most exist as accessory minerals because they are composed of rare elements or of elements that easily fit into other minerals. Magnetite and ilmenite, for example, are generally minor because they comprise elements that also easily fit into other more abundant essential minerals. Rocks that contain fluorine (F), zirconium (Zr), or phosphorus (P) may contain fluorite, zircon, or apatite, but F, Zr, and P are very minor elements in all but the most unusual rocks. Although not abundant, zircon often contains uranium and lead, which we may analyze to learn radiometric ages of igneous rocks.
6.5 Naming Igneous Rocks
There are a number of different ways to divide igneous rocks into groups and to give them names. Assigning names can be complicated because igneous rocks vary greatly in mineralogy and texture, and because rock types grade from one to another. If we invoke too many names, terminology becomes too complicated to be useful. If we invoke too few names, they are too broad to have much meaning. The task is somewhat simplified because, with rare exceptions, the major elements in all igneous rocks are the same. Additionally, certain minerals have affinities for each other because of their chemical compositions. Mafic igneous rocks, for example, generally contain pyroxene and Ca-plagioclase. Silicic igneous rocks contain predominantly quartz, alkali feldspar, and micas. Mafic rocks, therefore, have a characteristic dark color, while silicic rocks are white or pink. Despite these generalizations, however, igneous petrology is complex and the nomenclature is complicated.
6.5.1 Simple Classification Scheme
Most igneous rocks contain feldspar or quartz. So, the most widely used and relatively straightforward classification scheme for igneous rocks is based on the relative amounts of quartz, plagioclase, and alkali feldspar that are present. It is the International Union of Geological Sciences (IUGS) system. Figure 6.98 shows a version for normal plutonic rocks. Other minerals may be present, but using this system, only the ratios of quartz, plagioclase, and alkali feldspar determine the rock name. As an example, we call a rock that contains nearly equal amounts of quartz and plagioclase, and that contains no or very little alkali feldspar, tonalite.
This IUGS system works well for many plutonic igneous rocks, and we can often identify quartz, plagioclase, and alkali feldspar in the field – so it is very convenient. It does not work well for rocks that do not contain significant amounts of quartz or feldspar, and it makes no distinction between rock types that contain similar amounts of quartz, alkali feldspar, and plagioclase but vary significantly with respect to other minerals. For example, gabbro, diorite, and anorthosite all contain plagioclase, with very minor alkali feldspar and quartz, if any. They plot in the bottom right corner of the triangle and consequently cannot be distinguished.
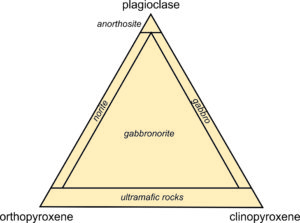
The main difference between gabbro and diorite is that gabbro, which is a mafic rock, contains calcium-rich plagioclase. Diorite, is more silicic than gabbro, and contains more sodic plagioclase. The standard IUGS system takes no account of feldspar composition, so this distinction is lost. Gabbro and anorthosite are closely related and compositions between are common. But, anorthosite is almost entirely plagioclase, and gabbro contains significant amounts of clinopyroxene in addition to plagioclase. The IUGS has developed a separate triangular diagram for gabbro and related rocks, it is shown here in Figure 6.99.
Magmas poor in SiO2 but rich in alkalis will yield rocks containing nepheline or leucite (both members of the feldspathoid group) instead of feldspars. Similarly, ultramafic rocks, that often contain no quartz or feldspars are not represented on the standard IUGS diagram. So, the IUGS has developed different triangular diagrams for these kinds of rocks.

The diagram in Figure 6.98 is for plutonic rocks. The IUGS has a similar system for volcanic rocks, but volcanic rocks may be very fine grained, making mineral identification difficult or impossible, and they may contain significant amounts of volcanic glass in lieu of minerals. So, instead of naming volcanic rocks based on mineralogy, petrologists often assign names based on alkali and silica content. This is the total-alkali-silica system (TAS), shown in Figure 6.100. The diagonal red line divides rocks into those that we call alkalic (above the line) and sub-alkalic (below the line). The most common volcanic rocks are sub-alkalic, ranging in composition from basalt (mafic) to rhyolite (silicic).
6.6 Mineral Modes
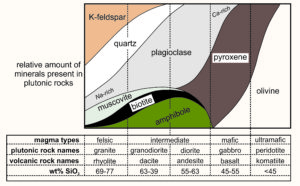
K-feldspar, quartz, plagioclase, pyroxene, olivine, muscovite, biotite, and amphibole are the most common minerals in igneous rocks. Figure 6.101 depicts the typical amounts by volume – also called the mineral modes – of these eight minerals in some common plutonic rocks. Volcanic rock names are included in the table, but many volcanic rocks contain glass that replaces some or all of the minerals that could be present. The table below gives more explicit information about the minerals common to different kinds of rocks.
Figure 6.101, and table below, emphasize the affinities that some minerals have for others. Silicic to intermediate rocks always contain K-feldspar and quartz and are more likely to contain hydrous minerals such as muscovite or biotite. They may contain Na-rich plagioclase. The most common mafic rocks contain pyroxenes and Ca-rich plagioclase. They may contain olivine, and almost never contain quartz or K-feldspar. Olivine, nepheline, and feldspathoid minerals can never coexist with quartz, because they would react to form other silica-rich minerals. Thus, certain minerals can be associated with each other in nature, while others cannot. The minerals present are primarily controlled by the composition of the magma, which in turn reflects the process and source that generated it. So, different igneous rocks, formed in different environments, have characteristic mineral assemblages.
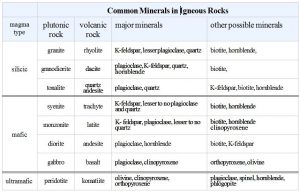
6.7 Common Types of Igneous Rock
6.7.1 Silicic Igneous Rocks (>20% Quartz)
Silicic igneous rocks (see the table above) include the plutonic rocks granite, granodiorite, and tonalite, and their volcanic equivalents (rhyolite, dacite, quartz andesite). The plate below contains examples of each. All contain > 20% quartz and we name them based on the amounts of K-feldspar and plagioclase they present. Biotite, hornblende, and muscovite also may be present as minor minerals. Some granitic rocks contain no plagioclase, and some tonalitic rocks contain no K-feldspar, but feldspars of some sort are always there. Plagioclase in granitic rocks may be nearly pure albite (the Na end-member), but in other silicic igneous rocks plagioclase is more Ca-rich. In extrusive rocks, K-feldspar may be sanidine instead of orthoclase or microcline, and cristobalite or tridymite may replace quartz. Silicic volcanic rocks often contain glass instead of some of the minerals.
| plutonic rocks |  |  |  |
| volcanic rocks |  |  |  |
| K-feldspar rich | both feldspars | plagioclase rich |
Because they contain large amounts of quartz and feldspars, granitic and granodioritic rocks have light colors. Granite (Figure 6.102) and rhyolite (Figure 6.105) may have a pinkish color due to oxidized Fe in K-feldspar. These rocks also commonly contain biotite or hornblende. Granodiorite (Figure 6.103) and dacite (Figure 6.106) usually have a grayish color. These rocks contain quartz, plagioclase, and K-feldspar. Often they have hornblende or biotite, too. (The granodiorite in Figure 6.103 contains lots of biotite.) Tonalite (Figure 6.104) and andesite (Figure 6.107) may be darker than the other four rocks because they contain lots of calcic plagioclase (which commonly has a darkish color) and often large amounts of biotite, hornblende, and, occasionally, pyroxene. Note the hornblende phenocrysts in the photo of andesite (Figure 6.107). Accessory minerals in all these rocks can be magnetite, ilmenite, rutile, pyrite, pyrrhotite, zircon, sphene, or apatite.
Identifying the different plutonic rocks is generally possible based on their macroscopic appearance, perhaps aided by a hand lens. But, telling the volcanic rocks apart is problematic without looking at thin sections using a petrographic microscope. Even then, it can be a challenge. In the field, when we do not have detailed chemical or mineralogical information, we often call pink volcanic rocks rhyolite, white ones andesite, and darker colored rocks basalt. Volcanic glass is very common, and if a lot of glass is present, naming a rock based on mineralogy is problematic.

Slowly cooling magmas do not crystallize all at once. After partial crystallization, the remnant melt may contain water and dissolved incompatible elements that did not enter any of the minerals already formed. When the remnant melt finally crystallizes, pegmatites containing minerals rich in incompatible elements such as potassium (K), rubidium (Rb), lithium (Li), beryllium (Be), boron (B), or rare earth elements (REEs) may result. Pegmatites often contain large euhedral crystals because the water acts as a flux and promotes crystal growth. Many spectacular and valuable mineral specimens come from pegmatites. Pegmatites are also the focus of much mining because of high concentrations of exotic elements they contain. The photo seen here (Figure 6.108) is a colorful tourmaline crystal from a pegmatite in Paraiba, Brazil. It is about 8 cm tall.
We saw other examples of pegmatite minerals in Chapter 4:
• Figure 4.12 – Riebeckite (an amphibole) with K-feldspar and quartz
• Figure 4.13 – Tourmaline similar to the one shown in Figure 6.108
• Figure 4.30 – Emerald (beryl) on quartz from Brazil
6.7.2 Mafic and Intermediate Igneous Rocks (0% to 20% Quartz)
Mafic rocks contain less that 5% quartz, if any. A dark color, due to an abundance of hornblende, clinopyroxene, or olivine, and sometimes Ca-rich plagioclase, characterizes these rocks. They include syenite, monzonite, and gabbro, and their volcanic equivalents trachyte, latite, and basalt. K-feldspar dominates syenitic rocks. Plagioclase is the only feldspar in most gabbros. Plagioclase composition varies from Na-rich in syenite to intermediate Ca-rich in gabbro. Hornblende, biotite, and pyroxene are common, but usually in small amounts.
Intermediate igneous rocks are those in which quartz accounts for 5% to 20% of the rock. They include quartz syenite, quartz monzonite, and diorite, and their volcanic equivalents quartz trachyte, quartz latite, and andesite. The minerals in these rocks are the same as listed in the previous paragraph for their more mafic cousins. As with silicic rocks, intermediate rocks with more plagioclase (diorite and some monzonite) tend to have more mafic minerals and thus a darker color.
While all intermediate and mafic igneous rocks may contain hornblende and clinopyroxene, hornblende is more common in diorite and andesite, and clinopyroxene is more common in gabbros and basalts. Gabbros and basalts may also contain olivine. Magnetite, ilmenite, and apatite are common as accessory minerals in mafic and intermediate rocks.
The photos below (Figures 6.109 to 6.116) show some of the most common intermediate and mafic rocks. They include four plutonic rocks:
• syenite from Brazil that contains the feldspathoid, nepheline
• monzonite that contains subequal amounts of plagioclase and K-feldspar with hornblende
• diorite that contains almost entirely plagioclase and hornblende
• gabbro that contains green olivine, light colored plagioclase, and darker clinopyroxene
The bottom row in the table contains photos of the volcanic equivalents of the four plutonic rocks above them:
• trachyte from the Massif Central of France (mostly K-feldspar and biotite)
• latite of unknown origin with subequal amounts of plagioclase and K-feldspar
• andesite (containing dark colored xenoliths) from New Mexico
• a view of basalt outcrops in Washington
| plutonic rocks |  |  |  |  |
| volcanic rocks |  |  |  |  |
| K-feldspar rich | both feldspars | plagioclase rich | ||
ppp
6.7.3 Ultramafic Igneous Rocks
Ultramafic rocks are especially poor in SiO2 and have high Mg:Fe ratios. Pyroxene (either orthopyroxene or clinopyroxene), olivine, and (less commonly) plagioclase are the dominant minerals in these rocks. The IUGS system, shown in Figure 6.117, names ultramafic rocks based on their relative amounts of olivine, orthopyroxene, and clinopyroxene. We call ultramafic rocks rich in olivine, peridotite. Those that are less than half olivine are pyroxenites. Other names, shown in the figure, divide peridotites and pyroxenites into smaller classes.
Many specimens of ultramafic rock are carried up from the mantle as xenoliths in magmas. The left and middle photos below (Figure 6.118 and 6.119) show examples. The photo on the left contains two different composition xenoliths. See the opening photo of this chapter for a larger view of this photo. The brown xenolith is mostly orthopyroxene and olivine. The green one contains light green olivine and darker green clinopyroxene, but they are hard to tell apart. The specimen also contains a few dark colored grains of orthopyroxene. Figure 6.119 shows a sample of garnet lherzolite that was carried to the surface in a South African kimberlite pipe. The garnets are red, olivine and orthopyroxene are green, and the less common emerald green grains are clinopyroxene. The photo on the right (Figure 6.120) shows a sample of pyroxenite, composed of large crystals of orthopyroxene and clinopyroxene. It is from the layered ultramafic Stillwater complex of Montana; two other photos of Stillwater rocks appeared earlier in this chapter (Figures 6.20 and 6.21).
 |  |  |
Ultramafic minerals are most stable at high temperature and they alter by reaction with water or carbon dioxide when exposed to normal Earth surface conditions. Consequently, finding fresh, unaltered ultramafic rock is difficult. Variable amounts of secondary serpentine, chlorite, talc, brucite, or calcite are nearly always present. We often find rocks called serpentinites, in which serpentine has replaced all or nearly all of the mafic minerals. Many ultramafic rocks – including those in kimberlite nodules – have been altered so much that we cannot determine their original mineralogy.
blank line
blank line
●Figure Credits
Uncredited graphics/photos came from the authors and other primary contributors to this book.
6.1 Xenoliths in basalt from San Carlos, Arizona, James St. John, Wikimedia Commons
6.3 Shiprock and one of the dikes radiating from it in New Mexico, el ui, Wikimedia Commons
6.4 Granite, amazon.com
6.5 Rhyolite, Amcyrus2012, Wikimedia Commons
6.6 Porphyry, Mike Norton, Wikimedia Commons
6.7 Pillow basalt, NamThai, Wikimedia Commons
6.8 Columbia River Flood Basalt, Washington, Walter Siegmund, Wikimedia Commons
6.9 Fifes Peaks, Washington, Ron Clausen, Wikimedia Commons
6.10 El Capitan in Yosemite Valley National Park, Mike Murphy, Wikimedia Commons
6.11 Mt. Whitney, Don Graham, Wikimedia Commons
6.12 Duluth Complex gabbro, James St. John, Wikimedia Commons
6.13 Nepheline syenite from Brazil, Amotoki, Wikimedia Commons
6.14 Vesicular olivine basalt from Hawaii, James St. John, Wikimedia Commons
6.15 Mt. Saint Helens May 1980, USGS, Wikimedia Commons
6.19 Chromite in the Bushveld Complex, Kevin Walsh, Wikimedia Commons
6.20 Olivine and ore from the Stillwater Complex, James St. John, Wikimedia Commons
6.21 Ore from the Stillwater Complex
6.22 Zoned tourmaline, Robert M. Lavinsky, Wikimedia Commons
6.23 Zoned clinopyroxene seen in thin section, www.ucl.ac.uk
6.26 Common quartz, Jon Zander, Wikimedia Commons
6.27 Euhedral quartz crystals, http://www.quartzpage.de/
6.29 Quartz in granite, Kevin Walsh, Wikimedia Commons
6.30 Arkose from the Garden of the Gods, Colorado, James St. John, Wikimedia Commons
6.31 Quartz Veins, Daniel Mayer, Wikimedia Commons
6.32 Amethyst in geode, 7iago1990, Wikimedia Commons
6.35 The composition of the Grorud feldspar
6.37 Albite from the Ziller Valley, Didier Descouens, Wikimedia Commons
6.38 Anorthite from Mt. Vesuvius, Robert M. Lavinsky, Wikimedia Commons
6.39 Orthoclase from Minas Gerais, Didier Descouens, Wikimedia Commons
6.40 Microcline from Araçuai, Brazi, Eurico Zimbres, Wikimedia Commons
6.41 Twinned sanidine, France, Didier Descouens, Wikimedia Commons
6.43 Polysynthetic twinning in feldspar, imgur.com
6.44 Carlsbad twin, mineralauctions.com
6.45 Baveno twin, mcdougallminerals.com
6.46 Mannebach twin, Didier Descouens, Wikimedia Commons
6.47 Twinning in microcline, www.alexstrekeisen.it, Wikimedia Commons
6.49 Fat separating from chicken broth with cooling, thepauperedchef.com
6.50 Perthite, Siim Sepp
6.51 Labradorite, Mjeltsch, Wikimedia Commons
6.58 Analcime from Martinique, Didier Descouens, Wikimedia Commons
6.59 Leucite, Géry Parent, Wikimedia Commons
6.60 Nepheline, Andrew Silver, Wikimedia Commons
6.61 Sodalite, pyrite, and-calcite, Géry Parent, Wikimedia Commons
6.62 Muscovite and Albite, Robert M. Lavinsky, Wikimedia Commons
6.63 Biotite on calcite, Didier Descouens, Wikimedia Commons
6.64 Phlogopite with calcite, Géry Parent, Wikimedia Commons
6.65 Margarite, Robert M. Lavinsky, Wikimedia Commons
6.66 Kyanite, staurolite, and Paragonite, Robert M. Lavinsky, Wikimedia Commons
6.67 Lepidolite, Eurico Zimbres, Wikimedia Commons
6.69 Clinochlore from West Point, New York, Robert M. Lavinsky, Wikimedia Commons
6.73 Jadeite from California, greatmining.com
6.74 Yellow spodumene, Géry Parent, Wikimedia Commons
6.76 Enstatite crystals, Leon Hupperichs, Wikimedia Commons
6.78 Augite from Rwanda, Didier Descouens, Wikimedia Commons
6.80 Microscope view of exsolution in a pyroxene grain, www.alexstrekeisen.it
6.83 Anthophyllite from eastern Finland, Leon Hupperichs, Wikimedia Commons
6.84 Typical green blades of actinolite, plugin.us
6.85 Tremolite, Didier Descouens, Wikimedia Commons
6.86 Hornblende, James St. John, Wikimedia Commons
6.87 Amphibolite from Huelva, southwestern Spain, PepPEfe, Wikimedia Commons
6.88 Glaucophane with omphacite, James St. John, Wikimedia Commons
6.89 Beryl, Nkansah Rexford, Wikimedia Commons
6.90 Tourmaline, Jan Helebrant, Wikimedia Commons
6.91 Epidote from Prince of Wales Island, Didier Descouens, Wikimedia Commons
6.92 Zoisite from Tanzania, Robert M. Lavinsky, Wikimedia Commons
6.93 Lawsonite, Kelly Nash, Wikimedia Commons
6.96 Olivine basalt, Simm Sepp, Wikimedia Commons
6.97 Olivine nodule Zbynek Burival, Wikimedia Commons
6.102 Granite, amazon.com
6.103 Granodiorite from Victoria, Australia, Solarence, Wikimedia Commons
6.104 Tonalite from Germany,Vberger, Wikimedia Commons
6.105 Rhyolite, Amcyrus2012, Wikimedia Commons
6.106 Dacite from Lassen Volcanic National Park, National Park Service
6.107 Quartz andesite with hornblende phenocrysts, cejones, pitt.edu
6.108 Tourmaline from Paraiba, Brazil, Géry Parent, Wikimedia Commons
6.109 Nepheline syenite in Rio de Janeiro, Amotoki, Wikimedia Commons
6.110 Monzonite, NASA, Wikimedia Commons
6.111 Diorite from Massachusetts, Amcyrus2012, Wikimedia Commons
6.112 Olivine gabbro, Randolph Black, Wikimedia Commons
6.113 Trachyte from Le Mont Dore, France, Francoise Foliot, Wikimedia Commons
6.114 Latite, www.alexstrekeisen.it
6.115 Rhyolite, Amcyrus2012, Wikimedia Commons
6.116 Basalt columns in Mt. Rainier National Park, Walter Siegmund, Wikimedia Commons
6.118 Peridotite xenoliths from San Carlos, James St. John, Wikimedia Commons
6.119 Garnet lherzolite from South Africa, James St. John, Wikimedia Commons
6.120 Pyroxenite from the Stillwater Complex, James St. John, Wikimedia Commons
Central Oregon Light Colored Rock With Silica
Source: https://opengeology.org/Mineralogy/6-igneous-rocks-and-silicate-minerals/